common name: fruit-tree pinhole borer
scientific name: Xyleborinus saxesenii Ratzeburg, 1837 (Insecta: Coleoptera: Curculionidae: Scolytinae)
Introduction - Distribution - Description - Biology and Ecology - Hosts - Damage - Management - Selected References
Introduction (Back to Top)
The fruit-tree pinhole borer, Xyleborinus saxesenii Ratzeburg (Figures 1 and 2), is an ambrosia beetle and a member of the weevil tribe Xyleborini. In Florida, there are at least 30 species of ambrosia beetles, many of them non-native (Thomas 2007). Ambrosia beetles are characterized by having a symbiotic relationship with fungi. Most ambrosia beetles carry several types of ambrosia fungi, with a more abundant primary fungus and other, auxiliary fungi present in the mycangia (specialized storage pockets in the exoskeleton).
The primary fungal mutualist of Xyleborinus saxesenii is Raffaelea sulphurea Batra. Most ambrosia beetles do not feed on wood, but Xyleborinus saxesenii is an exception. The larvae feed on the “farmed” ectosymbiotic fungus growing in wood and the wood itself, classifying this species as xylomycetophagous, rather than just mycetophagous (feeding only on fungi) (Deyrup and Atkinson 1987). Xyleborinus saxesenii is attracted to ethanol-based baits exceptionally well, and often accumulates in ethanol-baited traps in numbers greater than other ambrosia beetles (Steininger et al. 2015).
Xyleborinus saxesenii was one of the first non-native scolytine beetles introduced to North America, probably around 100 years ago, from Europe or Asia. It is one of the most abundant scolytine beetles but is rarely damaging. Most cases where the species is reported as aggressive involve tree hosts in poor health (Rabaglia et al. 2006). This ambrosia beetle species has been recorded colonizing several woody plants (Crane et al. 2008, Rabaglia et al. 2006), stressed peach trees (Kovach and Gorsuch 1985), and dying pondspice (Fraedrich et al. 2011), swampbay, and avocado trees affected by laurel wilt (Carrillo et al. 2012).
Figure 1. Adult female Xyleborinus saxesenii Ratzeburg (lateral view). Photograph by Daniel Carrillo, Tropical Research and Education Center (TREC), University of Florida.
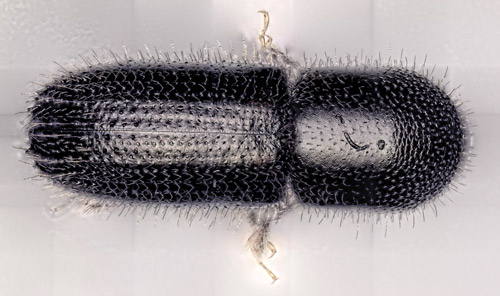
Figure 2. Adult female Xyleborinus saxesenii Ratzeburg (dorsal view). Photograph by Daniel Carrillo, Tropical Research and Education Center (TREC), University of Florida.
Although, the primary fungus is Raffaelea sulphurea (Harrington et al. 2010), Fraedrich et al. (2011) and Carrillo et al. (2014) reported the association of Xyleborinus saxesenii with Raffaelea lauricola T.C Harr, Fraedrich & Aghayeva, the causal agent of laurel wilt. According to Fraedrich et al. (2011) the association of Raffaelea lauricola and Xyleborinus saxesenii suggests that this beetle could be a vector. Nevertheless, the number of colony-forming units of Raffaelea lauricola recovered from the beetle was low compared to Xyleborus glabratus Eichhoff, the primary vector of the laurel wilt pathogen.
Distribution (Back to Top)
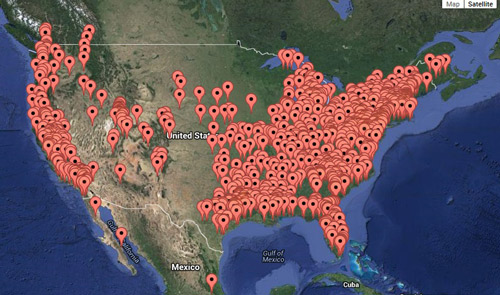
Xyleborinus saxesenii is distributed in temperate and subtropical regions worldwide (less commonly in tropical regions). The species is native to Eurasia, but has been introduced in Central America, South America, Africa, and Oceania. The beetle is believed to have been introduced to the U.S. via infested wood (Wood 1982). The beetle is present in most of the United States (Figure 3).
Figure 3. Current distribution of Xyleborinus saxesenii Ratzeburg in United States. Xyleborinus saxesenii is also present in South America and Asia, but this is not shown on this map. Atkinson 2015. http://www.barkbeetles.info/regional_chklist_target_species.php?lookUp=2037
Description (Back to Top)
Eggs: Eggs of Xyleborinus saxesenii are similar to eggs of other ambrosia beetles, less than 1 mm long and cloudy translucent or transparent (Figure 4). They are laid in groups of three to four inside the tunnel systems. The oval shape can help to distinguish them from the round eggs of mites sometimes present in the galleries.
Figure 4. Eggs of the fruit-tree pinhole borer, Xyleborinus saxesenii Ratzeburg. Photograph by Octavio Menocal, Tropical Research and Education Center (TREC), University of Florida.
Larvae: The larva of Xyleborinus saxesenii is similar to other ambrosia beetles. It is white to yellowish with a white-golden-colored head capsule, and legless (Figure 5). The larva goes through three instars until it reaches the pupal stage. The larvae enlarge the tunnel systems by digging and removing waste (frass). Larvae also groom other colony members and the gallery walls to prevent the spread of contaminants (Biedermann et al. 2012).
Figure 5. Larvae of the fruit-tree pinhole borer, Xyleborinus saxesenii Ratzeburg. Note the dark staining of the wood caused by the mutualistic fungus Raffaelea sulphurea (Kovach and Gorsuch 1988). Photograph by Octavio Menocal, Tropical Research and Education Center (TREC), University of Florida.
Pupa: The pupa of Xyleborinus saxesenii is white and motionless (Figure 6). Pupation takes place in the same gallery systems as larval development.
Figure 6. Pupa of the fruit-tree pinhole borer, Xyleborinus saxesenii Ratzeburg. Note the wood has stained black, which is caused by the ambrosia fungus covering the gallery wall. Photograph by Daniel Carrillo, Tropical Research and Education Center (TREC), University of Florida.
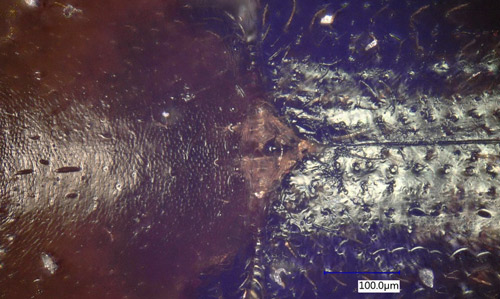
Adult: The adult of Xyleborinus saxesenii is less than 2 mm in length, elongate and cylindrical. The elytra are usually dark brown to black; the pronotum color ranges from light brown to dark brown. There is a distinct opalescence on the end of the elytra. Xyleborinus saxesenii is similar to Xyleborinus gracilis (Eichhoff) and several other Xyleborinus species in Florida (none of them native) as well as to several Xyleborus species (i.e., Xyleborus ferrugineus (Fabricius) and Xyleborus volvulus (Fabricius)). The key character distinguishing the genus Xyleborinus from other similar genera is the conical scutellum, positioned inside a notch at the base of the elytra, surrounded by a tuft of setae (Kovach and Gorsuch 1985, Figure 7). The species Xyleborinus saxesenii can be distinguished from two other locally common Xyleborinus by the absence of large spines on the end of the elytra (Xyleborinus gracilis has large spines) and by the rounded abdomen (Xyleborinus andrewesii (Blandford) has a tapering abdomen) (Figure 8).
Figure 7. The genus Xyleborinus can be distinguished from other similar genera by the shape of the scutellum, which is conical and positioned inside a notch at the base of the elytra, surrounded by a tuft of setae. Photograph by Daniel Carrillo, Tropical Research and Education Center (TREC), University of Florida.
Figure 8. Adults of the three Xyleborinus species common in Florida: Xyleborinus saxesenii Ratzeburg, Xyleborinus gracilis (Eichhoff) and Xyleborinus andrewesii (Blandford). Xyleborinus saxesenii can be distinguished from these Xyleborinus by the absence of large spines on the end of the elytra, which are present in Xyleborinus gracilis, and the shape of the abdomen, which is rounded rather than tapered as in Xyleborinus andrewesii (Blandford). Photograph by Daniel Carrillo, Tropical Research and Education Center (TREC), University of Florida.
Biology and Ecology (Back to Top)
Like many ambrosia beetles, Xyleborinus saxesenii has multiple generations per year. Adult females mostly attack the tree trunk rather than branches. Xyleborinus saxesenii is a haplodiploid species in which males develop from unfertilized eggs and are haploid, and females develop from fertilized eggs and are diploid. Eggs, larvae and pupae co-occur in the gallery system. There is usually only a single male in the brood, which is smaller than females and cannot fly. Females mate with their male siblings before leaving the natal gallery. Young females may delay dispersal and remain in the natal gallery engaged in mutually beneficial behaviors maintaining the fungus garden, therefore showing a “primitively eusocial” behavior (Biedermann et al. 2012). The flight behavior of Xyleborinus saxesenii has not been studied in detail but it may be similar to other scolytid species. In general, flight activity is initiated with an interaction of environmental cues such as relative humidity, light intensity, and temperature (Chen et al. 2010).
The available evidence suggests that females do not use aggregation or sex pheromones, instead they are attracted to volatiles emitted from plants. Xyleborinus saxesenii females follow long-range olfactory cues while in flight. As the female approaches the new host it probably relies on visual cues such as larger tree-diameter (Kendra et al. 2014). Preference for larger diameter trees could be related to the beetle fitness because larger trees can support larger beetle galleries, which is positively correlated with the number of beetle offspring (Mizuno and Kajimura 2002). Little is known about short-range cues, which could be a complex of different signals (gustatory, tactile, visual, olfactory, and chemosensory) that act synergistically (Kendra et al. 2014). A single, mated female can establish a new population (Biedermann et al. 2012).
Hosts
Xyleborinus saxesenii is capable of breeding in various hosts including members of the following families: Anacardiaceae, Annonaceae, Apocynaceae, Betulaceae, Cornaceae, Ericaceae, Fagaceae, Juglandaceae, Lauraceae, Magnoliaceae, Rosaceae, Salicaceae, Sapindaceae, Taxodiaceae, Tilicaceae, and Ulmaceae.
Damage
Like other ambrosia beetles Xyleborinus saxesenii breeds mostly in dead or dying trees. There are also reports of it attacking healthy chestnut (Oliver and Mannion, 2001) and stressed peach trees (Kovach and Gorsuch 1985). Xyleborinus saxesenii constructs galleries in the sapwood and damages the tree (Figure 9). Initially small strings of compacted sawdust protrude from the beetle entry holes. The sawdust strings fall to the ground (Figure 9), and a fine, white dust can be found at the base of the tree and in the bark cracks. If the bark is removed from the tree, the entrance holes of Xyleborinus saxesenii can be observed surrounded by a fungal stain. As the gallery is established, larvae begin to feed on the mixture of wood and fungus. As a consequence, the gallery system of Xyleborinus saxesenii (and other Xyleborinus) is irregular and resembles flat cavities rather than simple tunnels as in most other Xyleborini (Roeper 1995).
Figure 9. Damage caused by the fruit-tree pinhole borer, Xyleborinus saxesenii Ratzeburg, in avocado, Persea americana Mill. Photograph by Octavio Menocal, Tropical Research and Education Center (TREC), University of Florida.
Seasonality (Back to Top)
The seasonality of ambrosia beetles can be influenced by environmental cues such as: temperature, humidity, light intensity, and rainfall frequency (Rudinsky and Schneider 1969). According to Steininger et al. (2015), Xyleborinus saxesenii is one of the first ambrosia beetles to emerge in the spring in northern Florida. Weber and McPherson (1991) reported a flight activity for Xyleborinus saxesenii during the year with two flight peaks: late May and August-September in a North Carolina black walnut plantation. Our own unpublished data recorded flight peaks between February and April (in forest) in northern Florida and May through June (in avocado orchards) in southern Florida.
Management (Back to Top)
Xyleborinus saxesenii has been considered an unimportant pest until recently, when it has been shown to be able to spread the laurel wilt pathogen. No active management is needed unless associated with laurel wilt-infected trees. In order to avoid any risk of spreading the pathogen to new areas, infected trees must be cut, uprooted, chipped, and destroyed in the affected area. This prevents movement of infested wood and in some instances is legally required (Spence et al 2013).
Selected References (Back to Top)
- Atkinson TH. (2015). Bark ambrosia beetles of Canada and U.S. (Continental N. America). http://www.barkbeetles.info/regional_chklist_target_species.php?lookUp=2037 (22 March 2016)
- Biedermann PHW, Klepzig KD, Taborsky M, Six DL. 2012. Abundance and dynamics of filamentous fungi in the complex ambrosia gardens of the primitively eusocial beetle Xyleborinus saxesenii Ratzeburg (Coleoptera: Curculionidae, Scolytinae). Federation of European Microbiological Societies. Microbiology Ecology 83: 711-723.
- Carrillo D, Duncan RE, Peña JE. 2012. Ambrosia beetles (Curculionidae: Scolytinae) that breed in avocado wood in Florida. Florida Entomologist. 95: 573-579.
- Carrillo D, Duncan RE, Ploetz JN, Campbell AF, Ploetz RC, Peña JE. 2014. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathology 63: 54-62.
- Chen H, Li Z, Tang M. 2010. Laboratory evaluation of flight activity of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Canadian Entomologist 142: 378-387.
- Crane JH, Peña JE, Osborne JL. 2008. Redbay ambrosia beetle-laurel wilt pathogen: A potential major problem for the Florida avocado industry. Electronical Data Information Source. https://edis.ifas.ufl.edu/hs379 (12 December 2015).
- Deyrup M, Atkinson TH. 1987. Comparative biology of temperate and subtropical bark and ambrosia beetles (Coleoptera: Scolytidae, Platypodidae) in Indiana and Florida. The Great Lakes Entomologist 20: 59-66.
- Fraedrich SW, Harrington TC, Rabaglia RJ, Ulyshen MD, Mayfield III AE, Hanula JL, Eickwort JM, Miller DR. 2008. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Disease 92: 215-224.
- Fraedrich SW, Harrington TC, Bates CA, Johnson J, Reid LS, Best GS, Leininger TD, Hawkins TS. 2011. Susceptibility to laurel wilt and disease incidence in two rare plant species, pondberry and pondspice. Plant Disease 95: 1056-1062.
- Harrington TC, DN Aghayeva, SW Fraedrich. 2010. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111: 337-361.
- Kovach J, Gorsuch CS. 1985. Survey of ambrosia beetle species infesting South Carolina peach orchards and a taxonomic key for the most common species. Journal of Agricultural Entomology 2: 238-247.
- Kovach J, Gorsuch CS. 1988. Response of young peach trees to Ambrosiella sulphurea, a symbiotic fungus of Xyleborinus saxesenii. Plant Disease 72: 225-227.
- Mizuno T, Kajimura H. 2002. Reproduction of the ambrosia beetle, Xyleborus pfeili (Ratzeburg) (Col., Scolytidae), on semi-artificial diet. Journal of Applied Entomology 126: 455-462.
- Oliver AB, Mannion CM. 2001. Ambrosia beetle (Coleoptera: Scolytinae) species attacking chestnut and captured in ethanol-baited traps in middle Tennessee. Environmental Entomology 30: 909-918.
- Peer K, Taborsky M. 2007. Delayed dispersal as a potential route to cooperative breeding in ambrosia beetles. Behavioral Ecology and Sociobiology 61: 729-739.
- Rabaglia RJ, Dole SA, Cognato AI. 2006. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring North of Mexico, with an illustrated key. Annals of the Entomological Society of America 99: 1034-1056.
- Roeper RA. 1995. Patterns of mycetophagy in Michigan ambrosia beetles. Michigan Academic 27: 153-161.
- Rudisky JA, Schneider I. 1969. Effects of light intensity on the flight pattern of two Gnathotrichus (Coleoptera: Scolytidae) species. The Canadian Entomologist 101: 1248-1255.
- Spence DJ, Smith JA, Ploetz R, Hulcr J, Stelinski LL. 2013. Effect of chipping on emergence of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) and recovery of the laurel wilt pathogen from infested wood chips. Journal of Economic Entomology 160: 2093-2100.
- Steininger MS, Hulcr J, Šigut M, Lucky A. 2015. Simple and efficient trap for bark and ambrosia beetles (Coleoptera: Curculionidae) to facilitate invasive species monitoring and citizen involvement. Journal of Economic Entomology 108: 1115-1123.
- Thomas MC. 2007. Two Asian ambrosia beetles recently established in Florida (Curculionidae: Scolytinae). Pest Alert. Florida Department of Agriculture and Consumer Services. Division of Plant Industry, DACS-P-01647. (no longer available online)
- Weber BC, McPherson JE. 1991. Seasonal flight pattern of Scolytidae (Coleoptera) in black walnut plantations in North Carolina and Illinois. The Coleopterists Bulletin 45: 45-56.
- Wood SL. 1982. The bark and ambrosia beetles of North and Central America. A taxonomic monograph. Great Basin Naturalist Memoirs. Number 6. Brigham Young University. Provo, Utah. 1359 pp.