common name: giant black water beetle, giant water scavenger beetle (suggested common names)
scientific name: Hydrophilus triangularis Say (Insecta: Coleoptera: Hydrophilidae)
Introduction - Distribution - Description - Economic Importance - Cultural Importance - Selected References
Introduction (Back to Top)
The giant black water beetle (Hydrophilus triangularis) (Figure 1) is the largest aquatic-dwelling beetle in not only Florida, but in the entire United States (Epler 2010). Commonly seen across the continental US, this beetle has the widest distribution in the genus Hydrophilus (Short and McIntosh 2014). This beetle needs fresh water to reproduce, and prefers to dwell in large, deep ponds (Matta 1974). Hydrophilus triangularis, like other species within the family Hydrophilidae, is often seen on lighted sidewalks at night.
The larvae prey upon small invertebrates such as insects and snails, but can also consume tadpoles and small fish (Cranshaw 2010). As adults, these beetles will scavenge on decaying plant material and detritus in fresh bodies of water. In addition to scavenging plant material, Hydrophilus triangularis adults are predators on smaller aquatic insects, small fish, and snails (Hallmark 1972, Wilson 1923).
The adult beetles have an adaptation that allows them to surfacing less often for air than their larvae. The adults will hold an air bubble under their elytra (hardened wing covers) that they can use while underwater. The spiracles (tracheal openings) connect to this air bubble, and oxygen can be accessed when needed (Wilson 1923). The beetles can also store oxygen between small hairs along the body (Cranshaw 2010). This air supply is regenerated from the oxygen within the water, so frequent trips to the surface are not as necessary (Cranshaw 2010). When beetles do need to exchange the air in the bubble, they rise to the surface and make contact with the air using their long maxillary palps.
Figure 1. Adult giant black water beetle, Hydrophilus triangularis Say (dorsal view). Adult males reach up to 40 mm in body length. Photograph by Salvador Vitanza, Texas A&M University.
Distribution (Back to Top)
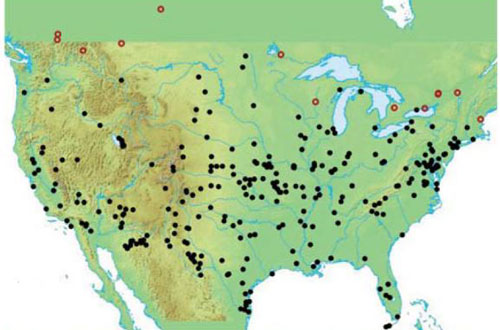
Hydrophilus triangularis beetles are found in Mexico, Canada, and the majority of states in the continental US (excluding Maine, Minnesota, New Hampshire, Vermont, and Wyoming) (Short and McIntosh 2014). Figure 2 represents the distribution of the beetle, which has the widest range within the genus north of Mexico (Short and McIntosh 2014).
Figure 2. Distribution of Hydrophilus triangularis Say beetles in the US and Canada. Black dots represent records checked by Short and McIntosh; red open circles represent only literature reports. Image from Short and McIntosh 2014.
Hydrophilus triangularis thrives in many areas due to its generalist feeding strategies. These beetles live through the winter, and mate and lay eggs in early summer (Wilson 1923). In warmer regions, the beetles may reproduce year-round, as resources for reproduction, such as an adequate food supply, are available during other seasons as well as summer. Their non-specific dietary needs contributes to their expansive range.
Description (Back to Top)
Eggs: The eggs of giant water beetles are about 5 mm long and deep yellow in color (Hallmark 1972). Female giant water beetles lay eggs in enclosed capsules made from silk. This strategy is common to most water-dwelling coleopterans. Females produce silk from accessory glands located in spinnerets on the underside tips of their abdomens (Beutel and Leschen 2016). The eggs are congregated together in the silk enclosure, and are deposited on floating debris in freshwater bodies (Cranshaw 2010). Composed of two layers, one layer of silk is attached to the floating substrate and one layer protects the eggs. The egg case facilitates a transfer of oxygen to the eggs and to the young larvae as they hatch (Beutel and Leschen 2016, Hallmark 1972). A unique projection, or mast, manifests on the egg casing as well. While the eggs develop, the mast ensures the case stays afloat by keeping it oriented upwards. It also keeps the case in a position to receive optimal air supply (Hallmark 1972).
Larvae: During all three instars of larval development, the immature beetles spend their time living in bodies of fresh water. Immature Hydrophilus triangularis larvae can walk along the bottom of a pond or even out in open air (Wilson 1923). Larvae breathe air through a tracheal system, and must surface occasionally to refresh their air supply before going back underwater (Wilson 1923). Larvae possess mandibulate (chewing) mouthparts that are slightly different in size and shape, and come together in a motion similar to scissors (Wilson 1923). This makes it difficult for actual chewing to occur, so the larvae can only bite, cut, or grab prey. Beetle larvae are a light yellowish-brown ventrally (underside) and a darker brown dorsally (backside). Two prominent lines run on either side of larvae down the length of the thorax to the tip of the abdomen (Wilson 1923). Larvae can swim using either their bodies and prolegs (fleshy appendages that serve as a larva’s legs) or walk using just the prolegs. Larval development usually lasts 18 to 22 days, with four days as first and second instars and the remainder in the third instar (Hallmark 1972).
Figure 3. Larval giant black water beetle, Hydrophilus triangularis Say. Larva attached to aquatic plant using prolegs. Photograph by Whitney Cranshaw, Colorado State University.
Pupae: As the larvae develop, they reach the pupal stage where they will remain until they molt into an adult. Larvae have aged enough to pupate (molt) once their color becomes a uniform dark brown on their dorsal and ventral sides (Wilson 1923). The larvae will then climb out of the body of water they have developed in and look for a place to bury themselves. Here they will dig a pupal cell, and will pupate (Hallmark 1972). The larvae typically travel between two and 10 feet from the water’s edge to dig their pupal cell (Wilson 1923). The larvae prefer soil consistency that is dense clay with a high moisture content, but will dig in sand if it is the only substrate available (Wilson 1923). The larvae dig the pupal chamber with their mandibles, which takes them between 36 and 48 hours to excavate (Wilson 1923). They use a rotating motion to scoop the sediment until they have a semi-circular hole that is about three inches deep (Wilson 1923). The larvae then insert themselves into the pupal cell, and pupate. Pupal development usually takes up to 10 days (Hallmark 1972) before adult emergence. During molting, the pupae resemble grubs, and are fleshy white with dark eyes (Hallmark 1972).
Figure 4. Pupal giant black water beetle, Hydrophilus triangularis Say (ventral view). Image from Wilson 1923.
Adults: Hydrophilus triangularis adults can be identified by their body shape and size. These beetles have a characteristic elongated and narrow body uncommon to other species. The largest beetle in the genus, Hydrophilus triangularis usually grows up to 40 mm (Cranshaw 2010). Adults can be distinguished from other members within the genus their protarsi, the last segment of an insect’s front leg. The protarsi of Hydrophilus triangularis have an ending sector that protrudes to form an obtuse angle rather than one at 90 degrees (see Figure 5) (Short and McIntosh 2014).
Figure 5. (Left) Protarsus of male Hydrophilus insularis Laporte. (Right) Protarsus of male Hydrophilus triangularis Say. Photographs by Oliver Keller, University of Florida.
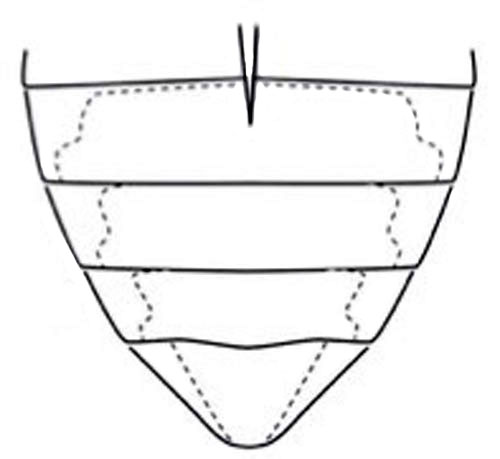
When looking at the exposed, sclerotized plates on the underside of a beetle’s body (abdominal ventritesof different species within the genus Hydrophilus, Hydrophilus triangularis has a pyramid-like region that is smooth and free from hairs or setae, whereas the rest of the abdominal ventrites possess hairs (Figure 6) (Short and McIntosh 2014). Other species tend to have reduced ventrites that are inverted in shape.
Figure 6. Line drawing of abdominal ventrites of Hydrophilus triangularis Say. Image from Short and McIntosh 2014.
Hydrophilus triangularis is commonly confused with the similar species Hydrophilus insularis. Both have adults that can reach lengths close to 40 mm, but Hydrophilus insularis is native to only four states in the US (Arizona, California, Florida, and Texas) (Short and McIntosh 2014). Hydrophilus insularis has an antennal cupule (cup-shaped antennomere) that is elongated to cover the first and second antennomeres that form the club. By contrast, that of Hydrophilus triangularis barely covers the antennal segment that first forms the club (Short and McIntosh 2014). Antennomere 1 is the scape, and is found closest to the head.
Economic Importance (Back to Top)
Hydrophilus triangularis is not classified as endangered or considered a pest, and is generally non-impactful to humans. The larvae and adults are predacious on small fish in ponds, but they do not pose a significant threat or cause imbalance to most aquatic food chains (Wilson 1923); however, this species of beetle has been found to be somewhat of a nuisance in commercial fish hatcheries, where it may feed on smaller fish (Arnett and Thomas 2001).
Because both mosquitoes (family Culicidae) and Hydrophilus triangularis require fresh water to breed, researchershave investigated whether this species could be used as a biological control to reduce mosquito populations. They found that a single Hydrophilus triangularis could successfully prey upon 194 larvae of Culex quinquefasciatus in only one day (Arnett and Thomas 2001). While Hydrophilus triangularis has not been implemented in any expansive project to control mosquitoes, it does appear likley to reduce mosquito populations in many freshwater areas.
Cultural Importance (Back to Top)
Although surprising to some, many people keep Hydrophilus triangularis as pets. Entomologists and bug enthusiasts may raise these beetles to see their life history progression or keep them as adults. Attractions like the Saint Louis Zoo keep these beetles in their insectarium as education for the public (Saint Louis Zoo ND).
Selected References (Back to Top)
- Arnett Jr RH, Thomas MC. 2001. American Beetles, Volume I: Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. CRC Press, Boca Raton, FL. 464 pp.
- Beutel RG, Leschen RAB. 2016. Coleoptera, Beetles. Volume 1: Morphology and Systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim) 2nd Edition. Hubert & Co. GmbH & Co. KG., Göttingen, Germany. 567 pp.
- Cranshaw W. 2010. Colorado Insects of Interest, Giant Water Scavenger Beetle. Center for Invasive Species and Ecosystem Health at the University of Georgia. Athens, GA. (22 January 2019)
- Epler JH. 2010. The Water Beetles of Florida - an identification manual for the families Chrysomelidae, Curculionidae, Dryopidae, Dytiscidae, Elmidae, Gyrinidae, Haliplidae, Helophoridae, Hydraenidae, Hydrochidae, Hydrophilidae, Noteridae, Psephenidae, Ptilodactylidae and Scirtidae. Florida Department of Environmental Protection, Tallahassee, FL. 399 pp.
- Hallmark MDM. 1972. The life history and life process studies of the water scavenger beetle, Hydrophilus triangularis Say (Coleoptera: Hydrophilidae). M.S. thesis dissertation, Texas Tech University. Lubbock, TX.77 pp.
- Matta JF. 1974. The Aquatic Hydrophilidae of Virginia (Coleoptera: Polyphaga). Virginia Polytechnic Institute and State University. Blacksburg, VA. 44 pp.
- Saint Louis Zoo. Undated. Giant Water Scavenger Beetle. St. Louis, MO. (22 January 2019)
- Short AEZ, McIntosh IV CE. 2014. Review of the giant water scavenger beetle genus Hydrophilus Geoffrey (Coleoptera: Hydrophilidae) of the United States and Canada. The Coleopterist’s Bulletin 68: 187-198.
- Wilson CB. 1923. Life History of the Scavenger Water Beetle, Hydrous (Hydrophilus) triangularis, and its Economic Relation to Fish Breeding. Bulletin of the Bureau of Fisheries. Fairport, IA. 38 pp.