common name: drone fly, rat-tailed maggot
scientific name: Eristalis tenax (Linnaeus) (Insecta: Diptera: Syrphidae)
Introduction - Distribution - Description - Life Cycle - Economic Importance - Management - Selected References
Introduction (Back to Top)
The family Syrphidae, also known as hover flies, flower flies, and syrphid flies, is one of the largest families of Diptera with over 5,000 described species (Capinera 2004). They are called hover flies because they can hover while in mid-flight, lingering in one place much like a hummingbird. While many Diptera are detrimental to humans, most syrphid flies are beneficial. The larvae of some species (for example, Allograpta obliqua) are natural predators of aphids, and many of the adults are important crop pollinators (Weems 1951).
Hover fly adults are often brightly colored and are commonly mistaken for the bees and wasps that they resemble (mimic) in appearance and behavior (Catts and Mullen 2002). Many are black with either white, yellow, or orange bands across the abdomen and similar in shape to bees and wasps. They also have similar flight behavior and feed on pollen and nectar (Brower and Brower 1965, Heal 1979). This type of mimicry is called Batesian mimicry because the mimic, although it is not dangerous to predators, benefits because the model is dangerous to predators (Bates 1961). In other words, predators may avoid bees, wasps and yellowjackets because they can inject toxins by stinging, so predators also avoid the flower flies that are mistaken for bees and wasps (Gilbert 1986).
Figure 1. Adult female hover fly, Chrysotoxum cautum (Harris), showing resemblence of some hover fly species to yellowjackets. Photograph by David A. Iliff, Cheltenham, England.
Figure 2. Adult female eastern yellowjacket, Vespula maculifrons (Buysson). Photograph by Bruce Marlin, http://cirrusimage.com.
The species Eristalis tenax (Linnaeus), commonly known as the drone fly (adult) or rat-tailed maggot (immature), is a mimic of the European honey bee Apis mellifera Linnaeus (Golding et al. 2001), and was introduced from Europe around 1875 (Gilbert 1986). The rat-tailed maggot is probably the source of Biblical writings that depict honey bees spontaneously developing from dead animals. This is because female drone flies can lay their eggs in carcasses (Osten-Sacken 1893).
Figure 3. Adult female drone fly, Eristalis tenax (Linnaeus). Photograph by Lloyd Spitalnik, Copyright 2006. www.lloydspitalnikphotos.com, Used with permission.
Figure 4. Adult honey bee, Apis mellifera Linnaeus. Photograph by Zachary Huang, http://www.beetography.com.
Adult E. tenax are important pollinators of many crops and wildflowers, while their larvae occasionally are pests around livestock (Day 2008) and cause accidental myiasis in humans (Catts and Mullen 2002). Myiasis occurs when fly larvae infest humans and other vertebrate animals and feed on the host's living tissue (Lakshminarayana et al. 1975), and is a common occurrence in certain other Dipteran species, such as bot flies (human bot fly, horse bot fly) and screwworms.
Distribution (Back to Top)
The rat-tailed maggot is cosmopolitan, occurring on every continent except Antarctica and ranges to the highest latitudes in the North (Metcalf 1913). It is absent in the extreme southern latitudes and in arid areas of Europe, Asia, and Africa (Thompson 1999). In the United States, it is found as far north as Alaska and south through California and Florida (Milne and Milne 1980).
Description (Back to Top)
Egg: The egg is white in color, has an elongate shape, and is covered in a sticky substance (Milne and Milne 1980).
Larva: The following information is from Metcalf (1913). The aquatic larva has a cylindrical shape with patches of horizontal folds dividing the body into segments, between which the cuticle is smooth. At the division of each body segment, two rows of flexible hairs are visible. The larva has a highly specialized organ on the posterior end (siphon) that acts as a respiratory appendage and also looks like a tail, thus giving them their nickname "rat-tailed maggot." The siphon can be several times the length of the body.
Figure 5. Larva of the rat-tailed maggot, Eristalis tenax (Linnaeus), about two and a half inches in length. Photograph by Walter Reeves, The Georgia Gardener.
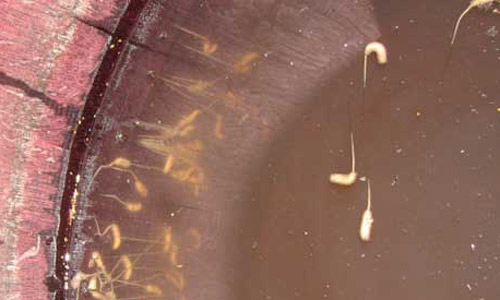
Figure 6. Larvae of the rat-tailed maggot, Eristalis tenax (Linnaeus), in dirty, polluted water within a tire. Photograph by Tami Ansley, Atlanta, GA.
Figure 7. Larvae of the rat-tailed maggot, Eristalis tenax (Linnaeus), in manure. Photograph by J. Keith Waldron, Cornell University.
Pupa: The pupa looks very similar to the larva but is shorter and thicker (Gilbert 1986). However, unlike the larva the pupa has two pairs of cornua, or horn-like bumps, located on the thorax (Metcalf 1913). The siphon remains present in the pupa but generally locks in a curved position over the back (Metcalf 1913).
Figure 8. Rat-tailed maggot pupa, Eristalis tenax (Linnaeus). Photograph by unknown.
Adult: The following information is from Milne and Milne (1980). The adult drone fly can be over half an inch in length. They can be easily differentiated from honey bees because they lack a constricted waist between the thorax and the abdomen, and they only have two wings, while honey bees have four. Short, brownish-yellow hairs are located on the thorax and the first segment of the abdomen. The body is dark brown to black in color, with yellow-orange marks on the side of the second abdominal segment while a narrow yellow-orange band crosses the third abdominal segment.
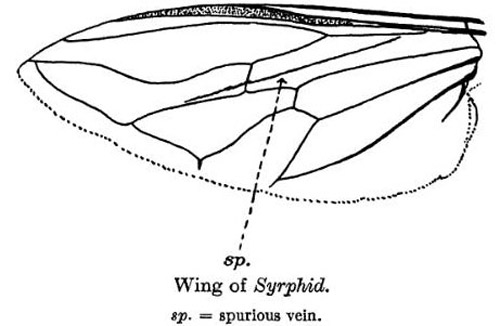
Like many other fly species, males can easily be distinguished from females because they have larger eyes that almost touch, while females have smaller eyes that are spaced further apart. Flies from the family Syrphidae can be distinguished from all other fly species by the identification of a spurious vein, or "false vein." This vein does not terminate at the end of the wing or at another vein but has a free end, and is not as sclerotized as the other wing veins (Metcalf 1913)
Figure 9. Adult male drone fly, Eristalis tenax (Linnaeus). Photograph by Lloyd Spitalnik, Copyright 2006. Used with permission.
Figure 10. Adult female drone fly, Eristalis tenax (Linnaeus). Photograph by Lloyd Spitalnik, Copyright 2006. www.lloydspitalnikphotos.com, Used with permission.
Figure 11. Spurious vein that is indicative of all Syrphidae flies. Illustration from Maxwell Lefroy, Manual of Entomology, 1923.
Life Cycle (Back to Top)
The drone fly undergoes complete metamorphosis with three larval instars. Usually two to three generations are produced each year (Gilbert 1986). However, there are many gaps in our understanding of the drone fly life cycle and more research is needed to provide detailed information on the life cycle.
Eggs: Eggs are deposited near the surface of foul water or decaying organic material, and are laid in masses with the eggs side by side, perpendicular to the ground (Metcalf 1913). It is not known how long it takes for eggs to hatch.
Larvae: Drone fly larvae are aquatic (Metcalf 1913), but sufficient solid food must be present to complete development, which is why they are found in water with high levels of organic matter (Day 2008). The respiratory appendage located posteriorly remains at the surface of the water while the larva moves through the water at various depths, allowing it to search for food without having to return to the surface to breathe (Metcalf 1913). Larvae are reported to reproduce by way of neoteny or paedogenesis, where each larva copies itself, reproducing from seven to 30 daughter larvae (Ibrahim and Gad 1978). However, there was only one observation of this occurrence.
Figure 12. Larvae of the drone fly, Eristalis tenax (Linnaeus), sharing their habitat with mosquito larvae. The drone fly larvae are represented by the dimples where their siphons are attached to the surface of the water. Photograph by Phillip E. Kaufman, University of Florida.
Pupae: Pupation occurs in a drier environment than where the larvae develop. This is usually just below the soil surface, where they remain for eight to 10 days (Gilbert 1986, Milne and Milne 1980). Cornua that appear on the pupa are thought to aid in respiration during the pupation period as the trachea within the siphon becomes unusable (Metcalf 1913).
Adults: Females will feed on pollen once they emerge from the pupa in order to obtain the necessary nutrients to complete reproduction (Gilbert 1986). Subsequent meals will consist mainly of nectar to provide the energy necessary for activity (Gilbert 1986). Adult drone flies often feed on nectar from daisies, chrysanthemums, and asters (Gilbert 1986). The adults prefer yellow flowers, leading to their importance in the pollination of yellow-flowered crops (Ilse 1949).
Male E. tenax tend to be territorial. Observations suggest that males may live in the same territory their entire lives where they mate, feed, and groom, defending this area against other insects (Wellington and Fitzpatrick 1981). Mating can occur while the pair is flying, with the male uppermost, or terrestrially while resting on foliage (Rogers and Walker 1916). After mating, adult rat-tailed maggot females lay clusters of about 20 eggs near dirty, contaminated water, sewage, or decomposing organic substances (Milne and Milne 1980).
Adults can be found from late March to early December and most often in September and October (Gilbert 1986). In the late autumn months, females from the latest generation will mate and find a secure place to overwinter. The sperm remain alive, nourished by fat reserves from the female, while her eggs remain undeveloped until the spring (Kendall and Stradling 1972). After overwintering, the female emerges and lays from 80 to 200 eggs, and the cycle begins again (Kendall and Stradling 1972).
Economic Importance (Back to Top)
Eristalis tenax is usually not a serious pest, but occasionally the larvae can become a nuisance in livestock areas, where they are often abundant in manure lagoons and holding pits (Kaufman et al. 2000). During the summer, larvae can migrate from these sites in massive numbers as they seek dry pupation sites (Day 2008). The migrations can cause many problems, such as contamination of livestock feed, short circuits from accumulations in electrical boxes, and congregations in barn stalls, egg cartons, and other unwanted places (Kaufman et al. 2000, Day 2008).
Figure 13. A dairy farm lagoon in upstate New York. Photograph by J. Keith Waldron, Cornell University.
Figure 14. Rat-tailed maggots of the drone fly, Eristalis tenax (Linnaeus), infesting a dairy farm lagoon in upstate New York. Photograph by J. Keith Waldron, Cornell University.
The drone fly is reported to have caused accidental myiasis, which occurs when fly larvae inhabit a living host by accident, usually because of the ingestion of contaminated food in humans (Lakshminarayana et al. 1975). This can transpire in four ways: intestinal or gastric, nasal, auricular, or anal, with intestinal or gastric being the most common (Rogers and Walker 1916, Herms 1969). The larvae are able to survive gastric fluids, possibly due to the fact that they are adapted to living in polluted habitats (Aguilera et al. 1999). Myiasis from E. tenax becomes apparent when the host notices the larvae in their bowel movements (Lakshminarayana et al. 1975, Aguilera et al. 1999). Symptoms of this condition include diffuse abdominal pain and diarrhea, and can easily be treated with medication which expels the larvae from the body (Lakshminarayana et al. 1975, Aguilera et al. 1999).
Management (Back to Top)
Because E. tenax larvae live in highly polluted water, lagoons and manure pits need to be kept in the best condition possible. Not allowing the manure to extend through the surface of the water can help prevent fly development (Lyon 1995, Day 2008). Physical blockades between lagoons and barns/coops can prevent larvae from migrating to the barns/coops when searching for pupation sites, and will keep the pupating larvae in a non-essential area (Lyon 1995, Day 2008). Agitating the lagoons frequently by pumping, especially during the warm summer months can disrupt larval development.
Selected References (Back to Top)
- Aguilera A, Cid A, Regueiro BJ, Prieto JM, Noya M. 1999. Intestinal myiasis caused by Eristalis tenax. Journal of Clinical Microbiology 37: 3082.
- Bates HW. 1862. Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae. Transactions of the Linnean Society 23: 495-566.
- Brower JZ, Brower LP. 1965. Experimental studies of mimicry. 8. Further investigations of honeybees (Apis mellifera) and their dronefly mimics (Eristalis spp.). The American Naturalist 99: 173-187.
- Capinera JL. 2004. Flies. pp. 875-883. In Encyclopedia of Entomology, Vol. 2. Capinera JL [ed.]. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- Catts EP, Mullen GR. 2002. Myiasis (Muscoidea, Oestroidea). pp. 319-348. In Mullen G, Durden L [eds.], Medical and Veterinary Entomology. Academic Press an Imprint of Elsevier, San Diego, CA.
- Day ER. (2008). Livestock area fly control. Virginia Tech Extension. https://ext.vt.edu/pubs/pmg/fc/LivestockAreaFlyControl.pdf (5 February 2009).
- Gilbert FS. 1986. Hoverflies. Cambridge University Press, Cambridge, England.
- Golding YC, Ennos AR, Edmunds M. 2001. Similarity in flight behaviour between the honeybee Apis mellifera (Hymenoptera: Apidae) and its presumed mimic, the dronefly Eristalis tenax (Diptera: Syrphidae). The Journal of Experimental Biology 204: 139-145.
- Heal J. 1979. Colour patterns of Syrphidae II. Eristalis intricarius. Heredity 43: 229-238.
- Herms WM. 1969. Herms's Medical Entomology 6th ed. James MT, Harwood RF [eds.], The Macmillan Company, London, England.
- Ibrahim IA, Gad AM. 1978. The occurrence of paedogenesis in Eristalis larvae (Diptera, Syrphidae). Journal of Medical Entomology 12: 268.
- Ilse D. 1949. Colour discrimination in the drone fly Eristalis tenax. Nature 163: 255-256.
- Kaufman PE, Rutz DA, Waldron JK. (2000). Common pest flies found in the urban/rural environment and their biological control agents. IPM Fact Sheet 1021PMFS1. Cornell Cooperative Extension. http://entomology.cornell.edu/Extension/Vet/PDF_Files/Common_pest_fly_factsheet.pdf (5 February 2009).
- Kendall DA, Stradling DJ. 1972. Some observations on the overwintering of the dronefly, Eristalis tenax (L.) (Syrphidae). Entomologist 105: 229-230.
- Lakshminarayana CS, Kanchana MV, Janakavalli R, Mallika M. 1975. Intestinal myiasis due to Eristalis tenax. Journal of the Indian Medical Association 65: 234-235.
- Lyon WF. (1995). Livestock and livestock building pest management bulletin 473. Rattailed maggots (Syrphid fly larvae). Ohio State Bulletin. http://ohioline.osu.edu/b473/b473_21.html (5 February 2009).
- Metcalf CL. 1913. The Syrphidae of Ohio. Ohio State University Bulletin 17: 1-123.
- Milne L, Milne M. 1980. The Audubon Society field guide to North American insects and spiders. Alfred A. Knopf, Inc., New York, NY.
- Osten-Sacken CR. 1893. On the oxen-born bees of the ancients (Bugonia), and their relation to Eristalis tenax, a two-winged insect. Bullettino della Societa Entomologica Italiana 25: 186-217.
- Rogers JS, Walker FW. 1916. Syrphidae of Maine. Maine Experimental State Bulletin 253.
- Thompson CE. (1999). Flower Flies. United States Department of Agriculture, The Diptera Site. http://www.sel.barc.usda.gov/diptera/syrphid/syrphid.htm (5 February 2009).
- Weems HV. 1951. Check list of the syrphid flies (Diptera: Syrphidae) of Florida. Florida Entomologist 34: 89-113.
- Weems HV. (2004). A hover fly, Allograpta obliqua (Say). Featured Creatures. http://entomology.ifas.ufl.edu/creatures/beneficial/hover_fly.htm (5 February 2009).
- Wellington WG, Fitzpatrick, SM. 1981. Territoriality in the dronefly. Eristalis tenax (L.) (Syrphidae). Canadian Entomologist 113: 695-704.