Steinernema yirgalemense
Nguyen, Tesfamariam, Gozel, Gaugler & Adams, 2005
(Prepared by Khuong B.
Nguyen)
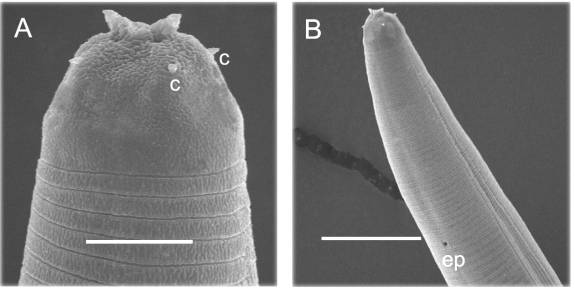
Infective juvenile head showing 2 horn-like structures
DESCRIPTION
Measurements
(Table 1)
First-generation male (Fig. 1, Fig. 2)
Body curved ventrally posteriorly, C-shaped when heat-relaxed. Head rounded, usually slightly swollen;
perioral disc not observed, six labial papillae, four larger cephalic papillae
and two amphids. Stoma shallow,
cheilorhabdions present as small and sclerotised structures at anterior end,
sometimes indistinct. Excretory pore
anterior to nerve ring, slightly posterior to middle of pharynx. Pharynx with cylindrical procorpus,
metacorpus absent or slightly swollen, isthmus present, nerve ring around
isthmus, just anterior to distinct basal bulb.
Pharyngo-intestinal valve present, usually weak. Gonad monorchic,
reflexed. Distance from base of pharynx
to anterior end of testis variable.
Spicules paired, brown in color. Head (manubrium) of spicules elongate,
length/width ratio ca 1.5 (1.4-1.7); shaft (calomus) present; blade
(lamina) thick, variable in shape tapering gradually posteriorly to a point;
velum prominent. Each spicule with two
internal ribs. Gubernaculum boat-shaped
in lateral view, gubernaculum neck short. Cuneus present but not
prominent. Twelve pairs of genital
papillae. Eleven pairs in normal position for Steinernema and 12 th
pair close to the edge of cloaca (Fig. 2C ) as reported for S. scapterisci
(Nguyen & Smart, 1992) and S. riobrave (Nguyen & Adams, 2003)
and one single precloacal genital papilla distributed as in Fig. 2B,C (6
preanal subventral [1-6] , one adanal [7], one lateral [11], 2 subterminal [8,
9] and one subdorsal [10]). Tail conoid; tail terminus without mucron.
Second-generation male
Second-generation
male is similar to first generation male but body size is smaller, spicule and
gubernaculum are shorter and thinner, excretory pore is much more anterior, and isthmus is narrower.
Mucron on tail terminus is rarely seen.
First-generation female (Fig. 3, Fig. 4)
Body cuticle smooth or with faint
annules under SEM. Lateral fields and phasmids not observed. Head rounded, continuous with body; six
labial papillae, four cephalic papillae. Lips indistinct. Amphids present. Stoma shallow,
subtriangular anteriorly; triradiate internally. Cheilorhabdions well sclerotised but small. A smaller sclerotised structure situated
posterior to cheilorhabdions (presumably the prorhabdions). Pharynx with procorpus cylindrical, muscular;
metacorpus slightly swollen; isthmus distinct; basal bulb valvate as in other
steinernematids. Nerve ring surrounding
isthmus, just anterior to basal bulb.
Pharhyngo-intestinal valve present.
Excretory pore located near middle of pharynx. Gonads amphidelphic,
reflexed, often containing many eggs. Vulva, a transverse slit; usually
protruding; low, thick epiptygma present in adult females. Vagina sclerotised,
short. Body diameter anterior and
posterior to vulva equal. Tail terminus
rounded, ventral postanal swelling present but small, tail shorter than anal
body diameter.
Second-generation female
Similar
to first generation female but smaller (length = 2385 μm, maximum diameter
= 149 μm compared to 6512 μm and 264 μm, for first-generation female).
Tail, tapering gradually to tail terminus, longer than anal body diameter;
terminal mucron present; ventral postanal swelling present.
Infective juvenile (Fig. 3, Fig. 5)
Body elongate. Sheath (second-stage cuticle) present immediately after
harvesting, but many IJ losing sheath in storage. Ensheathed juvenile with 6
labial and 4 cephalic papillae. Labial region smooth, continuous. Two horn-like structures present on exsheathed
juvenile; four prominent cephalic papillae.
Amphids prominent, located immediately below horns. Cuticle marked with prominent transverse
striations. Lateral field beginning anteriorly with one line, from annules 3 or
4. Two additional lines appearing at annules 20-24 to form two ridges. Near
excretory pore level, number of ridges in lateral fields increasing from two to
six. Two submarginal ridges appearing more posteriorly, making a total of eight
ridges, ie, maximum number in lateral field. Portion with eight ridges
is longest part of lateral field. In posterior third of body, two submarginal
ridges disappearing gradually, with only six ridges remaining in lateral field.
Near phasmid, six ridges in lateral field becoming two ridges. Formula of lateral field is 2, 6, 8, 6, 2.
Pharynx with thin corpus, basal bulb more or less elongate with visible valve.
Tail attenuate, tapering gradually.
Hyaline portion occupying about 54% (47-61) of tail length.
Type host and locality
Type
host unknown. The isolate was collected in an area with banana and sugarcane in
Yirgalem, Ethiopia, altitude 1660 m, latitude 6o46.221’ N, longitude
38o22.649’ E.
Type specimens
Holotype
(male, first generation): Isolated from haemocoel of Galleria mellonella
and deposited in the United States Department of Agriculture Nematode
Collection (USDANC), Beltsville, Maryland. Allotype (female, first generation):
Same data as holotype, deposited in the USDANC, Beltsville, Maryland.
Paratypes: Same data as holotype. Many
males and females of the first generation and several third-stage infective
juveniles in TAF deposited in USDANC Beltsville, Maryland; two males, and two
females of the first generation, and 10 third stage infective juveniles
deposited in the Florida Collection of Nematodes, Florida Department of
Agriculture and Consumer Services, Gainesville, Florida; one male and one
female of the first generation, and 10 infective juveniles deposited in the
California Collection of Nematodes, University of California Davis Nematode
Collection, Davis, California.
Cross hybridization tests
Males
and females of S. yirgalemense did not interbreed with S. abbasi and S. riobrave. In the control, males and females of the
each species mated and produced offspring.
Molecular characterization
The
alignment of the sequences of the ITS regions of six species in the ‘bicornutum
group’ (S. abbasi, S. bicornutum, S. ceratophorum, S. pakistanense, S.
riobrave and S. yirgalemense) is presented in Figure 6. The partial
18S (nucleotides 1-172 in the
alignment), 5.8S gene sequence (475-636), and 28S portion (992-1069) show
little variation among the six species. The ITS1 (17-474 in the alignment) and
ITS2 (637-991) regions are much more variable and provide most of the base
differences for species diagnosis
(Nguyen et al. 2001, Adams et al. 1998).
Additionally, the alignment shows that S. yirgalemense has 70 diagnostic characters (see explanation
in Table 2, Fig. 6) and differs from its sister taxon S. abbasi at 183
base pairs (bp) of the ITS sequence (Table 3).
The lengths of the amplified sequences (Table 2)
and pair wise distances also are different among the six species (Table 3). The lowest pair wise distances are
between S. yirgalemense and S.
abbasi, 0.19614 from 183 bp differences; the highest values are between S.
bicornutum and S. riobrave, 0.45455 from 420 bp difference. For D2/D3 sequences, the lowest pair wise
distances are between S. bicornutum and S. ceratophorum, 0.04478,
from 21 bp differences. The phylogenetic relationships among the 17 studied Steinernema
species are presented in Figure 6 (tree
length = 3036, CI = 0.5471, RI = 0.3972) and Figure
7 (tree length = 982, CI = 0.6368, RI = 0.7747). All species with
horn-like structures on the labial region comprise a monophyletic group by
analysis of both ITS and D2/D3 regions.
Steinernema yirgalemense
appears as the sister taxon to S. abbasi and the clade S.
abbasi + S. yirgalemense is
well supported by bootstrap analysis. Morphometric, morphological and DNA analyses show that S.
yirgalemense appears to be evolving
independently from its sister taxon S. abbasi.
Diagnosis and relationships
Steinernema
yirgalemense is characterised by of the presence of
horn-like structures in the labial region of the IJ, and is placed in the
‘bicornutum group’. The new species can be recognised by the IJ body length
averaging 635 (578-693) μm, EP = 51 (45-59) μm; tail length = 62
(57-67) μm and E% = 83 (67-90). Lateral field pattern variable, the
formula for the arrangement of ridges from head to tail is 2, 6, 8, 6, 2 (Fig.
5). The new species can be further recognised by spicule with prominent velum,
tapering to a pointed terminus (Fig. 2); gubernaculum with short neck; the male
SW and GS ratios (Table 1) and especially by the presence of 12 pairs of
genital papillae (compared to 11 in other species). The twelfth pair position
is at the edge of cloaca (Fig. 2).
The new species is characterised
genetically by sequence lengths of ITS regions (960 bp), the length of the ITS1
(270 bp) and ITS2 (284 bp), by composition of its sequence (Table 2) and by
numerous unique, derived nucleotide character states.
Steinernema
yirgalemense is different from all other species (except
for the ‘bicornutum group’) by the presence of horn-like structures in IJ
labial region. In the ‘bicornutum group’, the body length of the IJ (635
μm) is similar to that of S. riobrave (622 μm) but different
from that of S. abbasi, S. bicornutum, S. ceratophorum, S.
pakistanense and S. thermophilum (Table 4).
Tail length, c ratio, D% and E% of the IJ of the new species are also different
and can be used to distinguish between nematode species in this group. The
formula of the lateral field pattern (2, 6, 8, 6, 2) is unique, and can be
differentiated from the other two most similar species S. abbasi, (2, 6,
8, 7, 6, 2) and S. riobrave, (2, 7, 8, 6, 2). Males can be
differentiated from other steinernematids by the shape (Fig. 1, 2) and length
of spicules and gubernaculum (Nguyen & Smart, 1997) and D% = 58, and SW =
1.8 (Table 5). The new species has 12 pairs
of genital papillae with pair 12 located at the edge of the cloaca. It is
similar to S. riobrave (Nguyen & Adams, 2003) but differs from other
members of ‘bicornutum group’. Females can be distinguished from other species
by their small epiptygma (Fig. 4).
References
Adams, B.J., Burnell, A.M.
& Powers, T.O. (1998). A phylogenetic
analysis of Heterorhabditis (Nemata: Rhabditidae) based
on internal transcribed spacer 1 DNA sequence data. Journal
of Nematology 30, 22-39.
Cabanillas, H.E., Poinar,
G.O., Jr. & Raulston, J.R. (1994). Steinernema
riobravis (Rhabditida:
Steinernematidae) from
Texas. Fundamental and Applied Nematology 17,
123-131.
Elawad,
S., Ahmad, W. & Reid, A. (1997). Steinernema
abbasi sp. n. (Nematoda:Steinernematidae)
from the Sultanate of Oman. Fundamental and Applied Nematology 20, 435-442.
Ganguly,
S. & Singh, L.K. (2000). Steinernema
thermophilum sp. n. (Rhabditida:
Steinernematidae) from India. International Journal of Nematology 10,
183-191.
Nguyen, K.B. (1988). A new nematode parasite of mole crickets: its taxonomy,
biology and potential for biological control. Ph. D.
dissertation.
Gainesville, Florida, University of Florida, 154 pp.
Nguyen, K.B. & Adams,
B.J. (2003). SEM and systematic studies
of Steinernema abbasi Elawad et al., 1997 and
S. riobraveCabanillas
et al., 1994 (Rhabditida: Steinernematidae) Zootaxa 179, 1-10.
Nguyen, K.B. & Duncan, L.W. (2002). Steinernema
diaprepesi n. sp. (Rhabditida : Steinernematidae), a parasite of the citrus
root weevil Diaprepes abbreviatus (L) (Coleoptera : Curculionidae). Journal
of Nematology 34, 159-170.
Nguyen, K.B., Maruniak, J. & Adams, B.J. (2001). The diagnostic and phylogenetic utility of
the rDNA internal transcribed spacer sequences of Steinernema. Journal of Nematology 33, 73-82.
Shahina, F., Anis, M., Reid, A.P., Rowe, J. & Mapbool, M.A. (2001). Steinernema pakistanense sp. n. (Rhabditida: Steinernematidae) from Pakistan. International Journal of Nematology 11, 124-133.