
FACTORS INFLUENCING THE CONTROL OF MOLE CRICKETS
BY STEINERNEMA SCAPTERISCI
By
Khuong
B. Nguyen
Entomology
and Nematology Department
University of Florida
(From Khuong B. Nguyen Dissertation,
1988, University of Florida)

FACTORS INFLUENCING THE CONTROL OF MOLE CRICKETS
BY STEINERNEMA SCAPTERISCI
By
Khuong
B. Nguyen
Entomology
and Nematology Department
University of Florida
(From Khuong B. Nguyen Dissertation,
1988, University of Florida)
In host-parasite relationships, the host usually has some kind of mechanism to resist invasion by natural enemies. Contrarily, natural enemies have factors that help them overcome the resistant mechanisms of their hosts. One mechanism of resistance is that foreign bodies are encapsulated by the host they invade. When working with mole crickets and Steinernema scapterisci, I found mites present on the mole crickets, and since mites have been shown to eat nematodes in the soil, I wondered if these mites may help reduce the number of nematodes attached to mole crickets and thus help protect the mole cricket from invasion. Whether nematode-infected mole crickets die in the soil or on the soil surface is another factor which is important in the biological control of mole crickets. The above considerations led to the studies reported herein.
MATERIALS AND METHODS
Experiment 1: Observation of the encapsulation
of nematodes in a drop of hemolymph of mole crickets.
The purpose of this experiment was to see if nematodes would be encapsulated
by the blood cells of mole crickets when placed in a drop of their blood.
Mole crickets were washed free of soil by letting them swim for several
minutes in a container of tap water. Then the posterior portion of a mole
cricket was dipped in 30% hydrogen peroxide for 2 minutes, the cercus was
cut at the tip with sterilized scissors, and a drop of blood which oozed
from it was placed in a 35 x 10 mm petri dish. One third-stage infective
juvenile nematode, which had been surface-sterilized in merthiolate for
2 hours then rinsed three times in deionized sterilized water, was transferred
to the drop of blood. The nematode was observed continuously for about
10 minutes and photographs were made of each changing event. After the
10 minute observation period, the petri dish containing the nematode in
a blood drop was placed in a larger (50 x 15 mm) petri dish containing
a small amount of water and kept at 25 C. The experiment was replicated
10 times. Each replicate was observed every 15 minutes for 10 hours and
again after 20 hours.
Experiment
2: Observation of the encapsulation of nematodes in blood stream of mole
crickets.
The purpose of this experiment was to see if nematodes would be encapsulated
when placed in the blood stream of mole crickets. A mole cricket, previously
anesthetized by carbon dioxide, was dissected partially to remove the ventral
cuticle and the body organs. Then an incision was made in the dorsal diaphragm
and 5 nematodes were released at the site of the incision with some of
them inside and some outside the heart (the heart continued to beat during
the experiment). After 10 minutes, the nematodes were removed and transferred
to a drop of fresh blood and observed immediately under a compound microscope
(immediate observation is important to insure that any changes observed
occurred when the nematodes were in the blood stream of the mole cricket
instead of in the drop of blood). The experiment was replicated 3 times.
Experiment
3: Observation of the behavior of a nematode predator in soil, mites.
The purpose of this experiment was to see if a mite found on mole crickets
would feed on infective-stage juveniles of Steinernema scapterisci.
Mites were collected from mole crickets, and 10 of them placed in a petri
dish containing nutrient agar. The agar plate was examined every day to
see if the mites survived and reproduced. Infective-stage juveniles of
the nematode were released in the agar dish containing mites and observed
at intervals to see if the mites ate the nematodes.
Experiment
4: Observation of the behavior of nematode infected mole crickets in plexiglass
chambers.
The purpose of this experiment was to observe the movement of mole crickets
in soil and to determine if mole crickets infected with S. scapterisci
would die underground or on the soil surface. Observation chambers were
made from two pieces of plexiglass (1 m2) held 1 cm apart by a wooden frame
which enclosed all four sides. The space between the two pieces of plexiglass
was filled with a sandy soil and 8 mole crickets infected with S.
scapterisci were released in each chamber at the uppermost side of
the upright chambers. The experiment was replicated 4 times. Movement of
the mole crickets was observed, and the place where they died noted. After
all mole crickets died, another set of uninfected mole crickets was released.
The reason for releasing the second set of mole crickets was to see if
they made new tunnels or used the tunnels already made by other mole crickets.
RESULTS AND DISCUSSION
Experiment 1
Almost immediately after a third-stage juvenile nematode was placed in
a drop of blood, the blood cells began to adhere to the head and tail regions.
The more the nematode moved, the more cells attached until the nematode
became
totally encapsulated by blood cells.
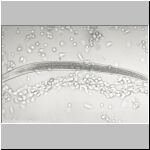

Different steps of the encapsulation
of infective juveniles of S. scapteriscii by hemocytes
of mole crickets
The cells around the nematode appeared to be held together by a thread-like substance which formed. The process of encapsulation took no more than 10 minutes, and the capsule remained around the nematode for the 10-hour period of observation. However, at the 20-hour observation period, 6 of the 10 nematodes had escaped from the capsule and moved freely in the drop of blood. In one of the capsules from which a nematode escaped, its shed cuticular sheath remained.
The
nematodes may have been able to escape from the capsules, because, as the
blood cells died, the cells lost the property that caused them to congregate
around a nematode and encapsulate it. In the case of the exsheathed cuticle
found encapsulated, it is likely that as the nematode exsheathed, it escaped
rather easily from the relatively few cells around the anterior end of
the old cuticle. Even though this may have happened in the drop of blood,
it is unlikely that it would happen in the body cavity of a mole cricket,
because, according to Poinar (1979), nematodes exsheath before or in the
process of entering the body cavity of an insect.
At the termination of the above experiment, it was performed many more
times to collect capsules for SEM observations. The capsules were collected,
transferred to a small glass petri dish containing water, and then prepared
for SEM observations according to the method of Stone and Green (1971).
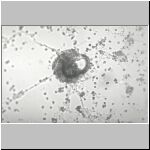
The SEM photographs
confirmed observations made through the light microscope.
SEM of nematodes encapsulated by blood cells
Experiment
2
The
nematodes removed from the blood inside and outside the heart of
a partially dissected mole cricket were partially
or totally encapsulated by blood cells. The cell layer (capsule)
was neither as thick nor as uniform as occurred in the drop of blood. The
capsule was thicker at the head and tail regions than along the body. Many
of the nematodes were encapsulated only over a part of the body, but the
experiment showed that encapsulation occurs in the blood stream of a mole
cricket as well as in an isolated drop of blood.
Experiment
3
After 5 days in an agar dish, mites grew quickly and different life stages
were observed. When third-stage juvenile nematodes were placed in the agar
dish containing mites, the mites consumed the nematodes readily. Mites
were observed to capture and consume a nematode in about 15 seconds.
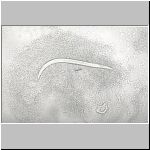
A nematode eaten
A nematode eaten
from anterior end
from posterior end
About 50 mites in one petri dish consumed about 2,000 nematodes overnight - an average of 40 nematodes/ mite. The mite was identified as Rhizoglyphus sp., family Acarididae, suborder Astigmata by Dr. Harvey L. Cromroy, Acarologist, Entomology and Nematology Department, University of Florida, Gainesville.
Experiment
4
Mole
crickets observed in the plexiglass chambers
moved
as deep as 70 cm below the soil surface. All of the infected
mole crickets died in their tunnels from 3 to 50 cm below the soil surface.
When uninfected mole crickets were placed in the chambers after the nematode-infected
ones died, they began to burrow into the soil, but when they ran across
a tunnel formed by the previous inhabitants, they tended to move in the
old tunnels instead of making new ones.
The
above experiments indicate that there are several factors which influence
the effectiveness of the nematode when used as a biological control agent
of mole crickets. The fact that mole crickets can
encapsulate invading nematodes increases their resistance to the
nematode, but the number of nematodes that a mole cricket can encapsulate
is not known. Neither do we know whether or not the bacterium is released
from an encapsulated nematode.
The presence of nematode-eating mites on the
body of mole crickets is another factor that can reduce the effectiveness
of the nematode. More studies are needed to determine the frequency
of occurrence of mites and their population density on mole crickets. Neither
do we know whether nematodes constitute a significant part of the diet
of the mites.
The fact that mole crickets died in the soil instead of on the surface,
and that they used the tunnels made previously by other mole crickets are
positive factors in biological control. Nematodes which emerge underground
from mole cricket cadavers have a much better chance of surviving long
enough to find a host than do those which emerge on the soil surface where
they are subject to desiccation and ultraviolet radiation. Since infected
mole crickets die in their tunnels and since other mole crickets use old
tunnels, the chance of nematodes and mole crickets encountering each other
are enhanced.