Steinernema glaseri
(Ssteiner, 1929) Wouts, Mracek, Gerdin & Bedding,
1982
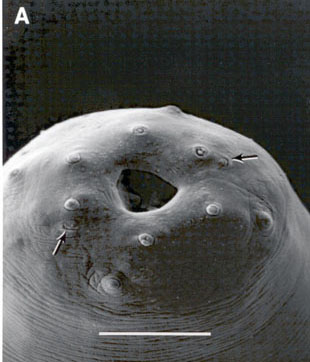
Face view of Steinernema glaseri
DESCRIPTION
Males: (FIG.1)
General morphology, same as the females. With a single ref1exed testis.
Spicule head short, shaft distinct, head comprise about 24% (21-25%) of
the spicule length (FIG.SEM),.
Blade long and narrow bearing two ridges. The distal tip of the spicules
bearing a ventral aperture which make it appear "hook- or "notch-like.
Velurn absent. Gubernaculum variable in shape, anterior end curved ventrally,
enlarged gradually posteriorly. The two wings of the corpus curved upward,
usually capitulum and cuneus forked anteriorly to form a Y-shape. Tail
has twenty-three genital papillae (eleven pairs and a single ventral preanal)
that are consistently present with little variation in position. Five of
the 11 pairs are preanal, subventral, pair six is lateral, pairs seven
and eight are subventral adanal (pair seven is sometimes preanal), pairs
nine and ten are subventral, subterminal, and pair eleven is subdorsal.
Measurements: Length=1700 micrometers
(um)(1500-1900), Width=72 um (54-92), anterior end to excretory pore=145
um (121-178), to nervering=132 um (99-183), esophagus length=160 um (155-187),testis
reflexion=176 um (84-264), tail=30 um (28-44), width at anus=42 um (34-47),spicule
length=77 um (62=90), spicule width=9 um (6-12), gubernaculum length=46
um (40-50), gubernaculum width=8 um (6-9). (After Poinar, 1978).
Females:(FIG.2),
Cuticle smooth, head slightly rounded. Six distinct lips united, each 1
papilla . Four cephalic papillae. Amphids crescent-shaped, narrow. Stoma
partially collapsed; cheilorhabdions represented by a thick ring of sclerotized
material just beneath the lips. Below this there isanother sclerotized
ring that represents the prorhabdions. Other part of stoma forming anasymmetrical
funnel with thick anterior end. Esophagus muscular with a cylindrical procorpus
followed by a slightly swollen non-valvated metacorpus, isthmus, and basal
bulb with a valve. The nerve ring is surrounding the isthmus just anterior
to tile basal bulb. Excretory pore opening usually anterior to nerve ring.
Lateral fields and phasmids inconspicuous. Gonads amphidelphic, reflexed.
Vulva a transverse slit from slightly to very protruding from the body
surface, with or without a thick flap. The vagina is short leading into
paired uteri. Eggs deposited initially, but they later hatch inside the
females and the juveniles bore their way out. First generation females
larger than those of the second generation. Tail with a prominent postanal
swelling, terminating with a rounded projection in the first generation
females and sometimes a fine mucro in second generation females of certain
isolates.
Infective juveniles:(Third
stage enclosed in second stage cuticle): The head is not annulated, labial
papillae not observed, four cephalic papillae and amphids pronounced. The
mouth and the anus are closed and the esophagus and intestine are collapsed.
The tail is conoid and slightly straight. The hyaline portion is < 1/2
of the tail length. The lateral field pattern begins anteriorly with one
line at the third annule, two lateral lines appear at annules 3-5 to form
two ridges (FIG.IJ3).
At the level of the isthmus and basal bulb, the number of ridges in the
lateral field increases from two to five and the lateral field is areolated.
A short distance posteriorly, the central ridge divides into two making
a total of six ridges in the lateral field, and areolation becomes less
obvious. More posteriorly, two additional lines appear on either side of
the lateral field to form two new ridges, making, a total of eight ridges,
the maximum in the lateral field. Near the level of the anus, the four
central ridges enlarge. Near the phasmid level the eight ridges in the
lateral field become two large bands or ridges. Occasionally, eight ridges
extend beyond the level of the phasmids and gradually reduced in width.
The phasmids are located near midtail. either ventral to the lateral fields,
or interrupting the ventral-most lateral ridges .
The two phasmids are located at almost the same level with a pore at the
center; the phasmids are often covered with exudate. No annules were observed
posterior to the phasmids on the ventral surface and only a few were observed
on the dorsal surface.
Measurements:Length=1130 um (864-1448),
width=43 um (31-50), anterior end to excretory pore=102 um (87-110), to
nerve ring=120 um (112-126), esophagus length=162 um (158-168), tail=78
um (62-87), a=29 (26-35), b=7.3 (6.3-7.8), c=14.7 (13.6-15.7), D%=65 (58-71),
E%=131 (122-138).
TYPE HOST AND LOCALITY
This nematode was first found in dead larvae of the Japanese
beetle (Popillia japonica) from Tavistock Golf Course near Haddonfield,
New Jersey (Glaser & Fox, 1930).
DISTRIBUTION AND
HOSTS
The nematode has been found later in Louisiana, Mississippi,
North Carolina (Poinar, 1979), Florida, Texas, Alabama (unpublished) in
the United States, and Santa Rosa, Brazil (Poinar, 1990). The nematode
attacks mainly soil insects, especially insects in the order Coleoptera
including the families Chrysomelidae, Curculionidae, Elateridae, Scarabaeidae.
The nematode also parasitizes some insects in the orders Lepidoptera (Poinar,
1979), Orthoptera (unpublished) and may be others.
BIONOMICS AND HOST
PARASITE RELATIONSHIPS
The life cycle of S. glaseri is similar to that
of other steinernematids, but this nematode develops more rapidly in Galleriamellonella
than others. It takes 3-4 days for S. glaseri to develop from infective
juveniles to adults compared to about five days for S. carpocapsae and
other species. This species is a tropical-origin nematode and survive well
between 15-35 oC (Kaya, 1990). When the infective juveniles of the nematode
are placed on the soil surface, most of the nematodes move downwards (Schroeder
& Beaver, 1987). When they are placed at the middle of a soil column,
more nematodes move downwards than upwards (Georgis & Poinar, 1983).
This nematode can survive better in sandy soil than soil with loam and
clay, because the nematodes may expend more energy to move in smaller pores
in loam and clay soil (Kung et al. 1990). When in soil, this nematode usually
moves in different direction to look for hosts. When a host is found, the
infective juveniles of S. glaseri enter the host body through the
mouth, spiracles or anus, then, into body cavity where they release bacteria
and develop as other steinernematids.
BACTERIAL ASSOCIATE
Infective stage of S. glaseri carry cells of Xenorhabdus
poinarii in their intestine. This bacterium was isolated and described
by Akhurst and Boemare (1988). Brown-pigmented, but intensity of pigmentation
is variable considerably between strains. This bacterium does not adsorb
bromothymol blue. Some strains produce antibiotic factors. In this species
there are more than two phases; adsorption of neutral red and production
of antimicrobial factors are not always associated. All strains grow at
37 oC while X. nematophilus from S. carpocapsae does not grow at this temperature.
BIOCONTROL CAPABILITY
Steinernema glaseri is the first nematode which
was investigated extensively as a biological control agent of insects.
Glaser (1932) produced the nematode in large numbers for the first time
by an in vitro method. At that time, Glaser did not know the bacteria associated
with infective juveniles, but his method was suitable for the development
of bacteria. The nematodes collected from his culture were applied in 73
field plots in New Jersey for control of Japanese beetle. Infected grubs
were recovered from 72/73 plots two weeks after application (Glaser, 1932,
Glaser et al. 1940) and the parasitization of the grub population in various
plots ranged from 0.3% to 81 % and the nematode remained in treated plots
for 8.5 years.
It has been shown that S. glaseri is very
effective as a biological agent of insects: in potted yews, the nematode
reduced more than 90 % of Japanese beetle (Wright et al. 1988). Georgis
and Hague (1991), who recently summarized fields trial against Japanese
beetle larvae in turf and pasture during the period 1984-1989, reported
that the application of S. glaseri reduced the insect population
equal to or better than the standard insecticide (Isofenfos). This nematode
also gave the best control of Adoryphorus couloni in Australia (Berg
et al. 1984, 1987).
LITERATURE CITED
Akhurst, R. J. & Boemare, N. E. (1988). A
numerical taxonomy study of the genus Xenorhabdus (Enterobacteriaceae)
and proposed elevation of the subspecies X. nematophilus to species. Journal
of General Microbiology 134:1835-1845. Berg, G. N. , Bedding, R. A.,Williams,
P., and Akhurst.,R. J. (1984). Developments in the application of the
nematodes for the control of subterranean pasture pests. Proceedings of
the 4th Australian Applied Entomology Research Conference, Adelaide: 352-356.
Berg,
G. N. , Williams, P., Bedding, R. A., & Akhurst, R. J. (1987).
A commercial method of application of entomopathogenic nematodes to pasture
for controlling subterranean insect pests. Plant Protection Quarterly 2:
174-177. Georgis, R & Hague, N. G. M. (1991). Nematodes as biological
insecticides. Pesticide Outlook 2:29-32. Georgis, R & Poinar G.
O. Jr. (1983). Effect of soil texture on the distribution of and infectivity
of Neoaplectana glaseri (Nematoda: Steinernematidae). Journal of Nematology
15:329-332. Glaser, R. W. (1932). Studies on Neoaplectana glaseri
, a nematode parasite of Japanese beetle (Popillia japonica). New Jersey
Department of Agriculture, Circular No. 211. Glaser, R. W. & Fox,
H. (1930). A nematode parasite of Japanese beetle (Popillia japonica
Newm.). Science 70:16-17. Glaser, R. W., McCoy, E. E. & Girth, H.
B. (1940). The biology and economic importance of a nematode parasitic
in insects. Journal of Parasitology 26:479-495. Kaya, H. K. (1990).
Soil ecology. Pp 93-115 in R.Gaugler and H. K. Kaya, eds. Entomopathogenic
nematodes in biological control. CRC Press, Boca Raton, Florida. Kung,
S. P. , Gaugler, R. & Kaya, H. K. (1990). Soil type and entomopathogenic
nematode persistence. Journal of Invertebrate Pathology 55:401-406. Nguyen,
K. B. & Smart, G. C. Jr., (1995). Scanning electron microscope
studies of Steinernema glaseri (Nematoda: Steinernematidae). Nematologica
41:183-190. Poinar, G. O., Jr. (1990). Taxonomy and biology of Steinernematidae
and Heterorhabditidae. Pp. 23-60 in R.Gaugler and H. K. Kaya, eds. Entomopathogenic
nematodes in biological control. CRC Press, Boca Raton, Florida. Schroeder,
W. J. & Beavers, J. B. (1987). Movement of entomopathogenic nematodes
of the families Heterorhabditidae and Steinernematidae in soil. Journal
of Nematology 19:257-259.
Wright, P. J., Noonan, M. J., Jackson T. A.
& Wouts, W. M. (1988). Use of nematodes for control of pasture
pests in New Zealand. Proceedings 5th Australasian Conference on Grassland
Invertebrate Ecology Melbourne: 82-87.
This document was constructed and is maintained by KHUONG
B. NGUYEN
Entomology & Nematology Department
University of Florida

