common name: robust crazy ant (suggested common name)
scientific name: Nylanderia bourbonica (Forel) (Insecta: Hymenoptera: Formicidae: Formicinae)
Introduction - Taxonomy - Synonymy - Distribution - Description - Biology - Life Cycle - Economic Importance - Selected References
Introduction (Back to Top)
Nylanderia bourbonica (Figure 1) is a widespread ant species that was introduced to the New World from the Old World tropics (Deyrup et al. 2000). This species, which has repeatedly and rapidly colonized subtropical and tropical islands around the world (Deyrup 2017), is the most widespread of its genus (AntMaps, Janicki et al. 2016). Nylanderia bourbonica is thought to be native to Southeast Asia. However, the species was first described from an introduced population on the French island of Réunion near the east coast of Madagascar (Deyrup 2017). The epithet bourbonica is derived from “Isle de Bourbon”, the original name of Réunion Island (Forel 1886).
Figure 1. Nylanderia bourbonica (Forel) worker. Photograph by Lyle J. Buss, University of Florida.
Taxonomy (Back to Top)
More than 130 species of ants belonging to the genus Nylanderia have been described worldwide. In addition to Nylanderia bourbonica, at least 14 species have been reported outside of their native ranges and are considered “tramp species” because they are spread by human activity and thrive around human habitation (AntMaps). Another species, Nylanderia vaga, is very similar in appearance to Nylanderia bourbonica; it is thought to have originated in Australasia and has since spread across the Old World tropics. Although Nylanderia vaga is not currently found in the New World, it is a pest species of concern that could thrive in Florida and other southern states. Distinguishing these two widespread pests remains challenging (LaPolla et al. 2011a), although it has been noted that Nylanderia bourbonica workers are distinctly larger than those of Nylanderia vaga (Wilson and Taylor 1967). While both species have dense pubescence (fine hairs) on the dorsal surface of the mesosoma (midsection of the body), Nylanderia bourbonica also has pubescence on the mesopleuron (sides of the mesosoma) while Nylanderia vaga does not (Sarnat and Economo 2012).
Two major obstacles have hindered accurate identification in this genus: (1) the need for a taxonomic revision of the many Nylanderia species, and (2) highly conserved morphology across the genus. The former has been at least partially addressed through regional revisions of the genus (LaPolla et al. 2011a, LaPolla et al. 2011b, Kallal and LaPolla 2012), but revisions for regions where Nylanderia are most speciose, including South America and southeast Asia/Australasia, have yet to be completed.
Synonymy (Back to Top)
Prenolepis nodifera bourbonica Forel (1886)
Prenolepis bourbonica Forel (1891)
Nylanderia (Nylanderia) bourbonica Forel (1912)
Paratrechina (Nylanderia) bourbonica Emery (1925)
Paratrechina bourbonica Trager (1984)
Nylanderia bengalensis (Forel) (1894)
Nylanderia bourbonica hawaiensis (Forel) (1899)
Nylanderia bourbonica skottsbergi (Wheeler) (1922)
Distribution (Back to Top)
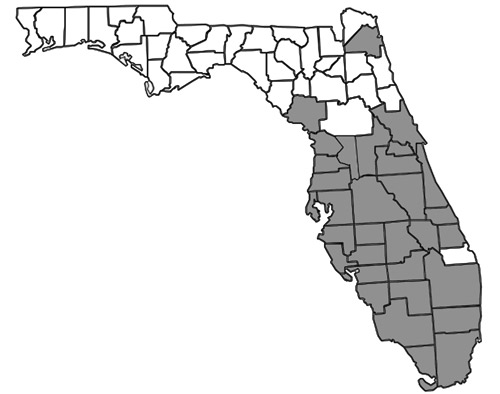
Nylanderia bourbonica is thought to be native to Australasia, but is also found in other tropical and subtropical regions worldwide, including Madagascar, Samoa, Hawaii, small islands in the Bahamas, and the southeastern United States (Deyrup 2017). In Florida, many records exist throughout the southernmost two-thirds of the peninsula (Figure 2). In the Florida Keys, Nylanderia bourbonica is well documented as a rapid recolonizer, capable of quickly re-establishing populations on experimentally defaunated islands (Simberloff and Wilson 1969, 1970). The first published record of the species in Florida was reported in 1930, but specimens have been found dating back to 1924 (Deyrup et al. 2000, Smith 1930). Historical records also exist from Mobile, Alabama (Trager 1984), though it is not clear when they were collected or whether there is currently an established population at this locality.
Figure 2. County distribution map of Nylanderia bourbonica (Forel) in Florida constructed using information from Deyrup (2017).
Description (Back to Top)
Worker: Adult workers (Figure 1) are 2.1-3.2 mm long and dark brown to almost black in color. Of the dark brown to black Nylanderia species found in the United States, Nylanderia bourbonica has the largest workers. In Florida populations, the legs (excluding the tarsal segments) and antennae are dark, but in the Pacific region the appendages tend to be lighter, and more yellow (Trager 1984). Overall the cuticle is smooth, glossy, and lacking surface sculpturing. Abundant erect, black, hair-like macrosetae and grey pubescence (fine hairs) are found on the head, mesosoma (midsection of the body), gaster, and legs, but no macrosetae are found on the propodeum (last segment of the mesosoma). Nylanderia species introduced to the Nearctic, including Nylanderia bourbonica, are known for having denser pubescence on the body than native species (Kallal and LaPolla 2012). The scape (first antennal segment) is covered in decumbent macrosetae. Workers of Nylanderia bourbonica are often confused with those of the white-footed ant (Technomyrmex difficilis). Both of these ants often cohabit the same disturbed sites in the field, but Nylanderia bourbonica is noticeably glossier, and Technomyrmex difficilis lacks the long, erect macrosetae on the mesosoma that are characteristic of Nylanderia species.
Head: The head is slightly longer than wide, and square in overall shape (Figure 3), with rounded posterolateral corners and convex lateral margins. The posterior margin is weakly concave to convex and varies between individuals. In all Nylanderia species, the eyes are positioned anterior to the midline of the head, a character useful for distinguishing them from Prenolepis, a closely-related genus (Williams and LaPolla 2016). The eyes of Nylanderia bourbonica are relatively large and weakly convex, but do not protrude beyond the lateral margins of the head. Three small ocelli are located medially, posterior to the eyes. The antennae are 12-segmented and long, and the scapes surpass the posterior margin of the head. The mandibles each have six teeth on the masticatory margin.
Figure 3. Frontal view of the head of a Nylanderia bourbonica (Forel) worker. Photograph from www.AntWeb.org. Photograph by April Nobile, California Academy of Sciences.
Body: The pronotum and mesonotum (dorsal parts of the first and second body segments, respectively) are convex, with the anterior edge of the mesonotum raised slightly above the pronotum. The propodeum is at about the same height as the pronotum, and rounded. In profile view, the petiole is forwardly-inclined and subtriangular in shape.
Figure 4. Profile view of Nylanderia bourbonica (Forel) worker. Photograph from www.AntWeb.org. Photograph by April Nobile, California Academy of Sciences.
Queen: Queen ants are morphologically similar to workers, but have expected modifications (Figure 5), including a robust mesosoma to accommodate flight muscles. They are dark brown and larger than workers, with a head that is wider than it is long, and very large eyes. Overall, the cuticle is finely punctate and covered in dense pubescence.
Figure 5. Profile view of Nylanderia bourbonica (Forel) queen. Photograph from www.AntWeb.org. Photograph by April Nobile, California Academy of Sciences.
Male: Male ants are similar to workers, but with expected modifications (Figure 6), including a robust mesosoma to accommodate flight muscles. Approximately the same size as workers (2.5-2.75 mm long), male Nylanderia bourbonica are dark brown to almost black and larger than any other dark-colored males found in Florida (Trager 1984). The head is much longer than wide, the eyes are very large, and the scapes are very long. The parameres (external genital lobes) of males are unique from those of other North American species in that they have a distinct dorsal notch (Kallal and LaPolla 2012).
Figure 6. Profile view of Nylanderia bourbonica (Forel) male. Photograph from www.AntWeb.org. Photograph by April Nobile, California Academy of Sciences.
Biology (Back to Top)
As generalist omnivores, Nylanderia bourbonica colonies primarily feed upon other insects for protein, and sugary exudates—including honeydew secretions from aphids and mealybugs—for carbohydrates. Like most other Nylanderia species, Nylanderia bourbonica workers are efficient and rapid foragers that recruit to resources quickly but have difficulty defending themselves from other species that arrive at the food source later (LaPolla et al. 2011a). For example, Nylanderia bourbonica is incapable of defending resources from the red imported fire ant, Solenopsis invicta (Trager 1984). However, another non-native Nylanderia species—the tawny crazy ant, Nylanderia fulva, is effective at outcompeting Solenopsis invicta through the deployment of defensive chemicals (LeBrun et al. 2013).
Nylanderia bourbonica colonies usually nest in soil and frequently move between temporary nesting sites that may only be habitable for up to a few weeks (Hölldobler and Wilson 1990). Nests are commonly associated with areas prone to frequent flooding. This species is most often found in naturally disturbed wet habitats—including mangroves and the edges of marshes and beaches—and although it is primarily a ground-nesting species, superficial nests associated with clumping vegetation or debris, such as driftwood, can also be found (Deyrup 2017).
Most of what is known about the reproductive biology of Nylanderia bourbonica is reported by Trager (1984). Winged reproductives, called alates, are produced by the colony year-round and can be found flying on warm mornings before the sun rises or after rainfall. Though copulation has never been observed, it is thought that mating occurs whilst the ants are airborne and prior to congregation at a light source, because females collected from lights consistently rear workers. After mating, females are attracted to reflective surfaces, including wet walkways and standing water. The reason for this could be that the females are susceptible to desiccation and require high humidity for survival.
Life Cycle (Back to Top)
All ants are holometabolous and undergo complete metamorphosis, with the following life stages (in order): egg, larva, pupa, and adult. Eggs are small, white, and cylindrical. Pupae are sometimes confused for eggs, but are distinguishable by a silk cocoon encasement and their relative size, which is about that of an adult; eggs are much smaller in comparison.
Economic Importance (Back to Top)
Unintentional introductions of “tramp species” of the genus Nylanderia have occurred since the early 1700s in ornamental plant imports and continue to occur today (Deyrup et al. 2000). Klotz et al. (1995) list Nylanderia bourbonica as the eighth most abundant urban pest ant in peninsular Florida. In Hawaii, Nylanderia bourbonica is one of several invasive species known to cause cosmetic damage to bananas with formic acid secretions (Nelson and Taniguchi 2012). Although several Nylanderia species are of major concern as pests, Nylanderia bourbonica is considered by Deyrup et al. (2000) to be a species of minor nuisance to outdoor eating areas that rarely enters buildings in large numbers, and presumably only does so to seek shelter during cold weather (Trager 1984).
According to Klotz et al. (1995), little is known about effective control of Nylanderia bourbonica. However, commercial liquid ant baits do attract workers and should be effective (Figure 7). Due to its minor pest status and infrequent indoor activity, broad application of pesticides should not be required. Instead, structural control is recommended through the sealing of possible entryways, especially around doors and windows, to prevent access to indoor areas. Since this species is known to inhabit damp areas, control of excess moisture may also aid in preventing infestation.
Figure 7. Nylanderia bourbonica (Forel) workers feeding on liquid ant bait. Photograph by Lyle J. Buss, University of Florida.
Selected References (Back to Top)
- Deyrup M. 2017. Ants of Florida: Identification and Natural History. Boca Raton: Crc Press.
- Deyrup M, Davis L, Cover S. 2000. Exotic ants in Florida. Transactions of the American Entomological Society 126: 293-326.
- Forel A. 1886. Études myrmécologiques en 1886. Annales de la Société entomologique de Belgique 30: 131-215.
- Hölldobler B, Wilson EO. 1990. The Ants. Cambridge: Harvard University Press.
- Janicki J, Narula N, Ziegler M, Guénard B, Economo EP. 2016. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecological Informatics 32: 185-193.
- Kallal RJ, LaPolla JS. 2012. Monograph of Nylanderia (Hymenoptera: Formicidae) of the World, Part II: Nylanderia in the Nearctic. Zootaxa 3508: 1-64.
- Klotz JH, Mangold, JR, Vail KM, Davis Jr. LR, Patterson RS. 1995. A survey of the urban pest ants (Hymenoptera: Formicidae) of peninsular Florida. Florida Entomologist 78: 109-118.
- LaPolla JS, Brady SG, Shattuck SO. 2011a. Monograph of Nylanderia (Hymenoptera: Formicidae) of the World: An introduction to the systematics and biology of the genus. Zootaxa 3110: 1-9.
- LaPolla JS, Hawkes PG, Fisher BL. 2011b. Monograph of Nylanderia (Hymenoptera: Formicidae) of the World, Part I: Nylanderia in the Afrotropics. Zootaxa 3110: 10-36.
- LeBrun EG, Abbott J, Gilbert LE. 2013. Imported crazy ant displaces imported fire ant, reduces and homogenizes grassland ant and arthropod assemblages. Biological Invasions 15: 2429-2442.
- Nelson S, Taniguchi G. 2012. Ant damage to banana fruits by abdominal secretions. General technical report IP-29. College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa. Honolulu, Hawaii.
- Sarnat EM, Economo EP. 2012. The Ants of Fiji. Berkeley: University of California Press.
- Simberloff DS, Wilson EO. 1969. Experimental zoogeography of islands: The colonization of empty islands. Ecology 50: 278-296.
- Simberloff DS, Wilson EO. 1970. Experimental zoogeography of islands: A two-year record of colonization. Ecology 51: 934-937.
- Smith MR. 1930. A list of Florida ants. Florida Entomologist 14: 1-6.
- Trager JC. 1984. A Revision of the genus Paratrechina (Hymenoptera: Formicidae) of the Continental United States. Sociobiology 9: 49-162.
- Williams JL, LaPolla JS. 2016. Taxonomic revision and phylogeny of the ant genus Prenolepis (Hymenoptera: Formicidae). Zootaxa 4200: 201-258.
- Wilson EO, Taylor RW. 1967. The ants of Polynesia (Hymenoptera: Formicidae). Pacific Insects Monograph 14: 1-109.