common name: Threecornered Alfalfa Hopper
scientific name: Spissistilus festinus (Say) (Insecta: Hemiptera: Membracidae)
Introduction - Synonymy - Distribution - Description and Identification - Life History - Hosts - Economic Importance - Management - Acknowledgement - Selected References
Introduction (Back to Top)
Spissistilus festinus (Figure 1) was first described by Thomas Say in 1831 and belongs to the insect order Hemiptera under the family Membracidae. The species’ common name “threecornered alfalfa hopper” was coined by farmers after observing large infestations in alfalfa. The presence of three corners on the pronotum (a prominent plate-like structure that covers all or part of the thorax of some insects), one at each shoulder and one at the apex, also confers the common name. Spissistilus festinus prefers to feed on leguminous plants and is a common insect in the southeastern United States (Wildermuth 1915).
Figure 1. Adult female three-cornered alfalfa hopper, Spissistilus festinus Say, (Lateral view). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
Synonymy (Back to Top)
Spissistilus festinus was first named Membracis festina in 1831 by Thomas Say. The genus was changed by Stal to Stictocephala and described as Stictocephala festina (Wildermuth 1915). Caldwell changed the tribe (taxonomic rank above genus) to Ceresini in 1949 and established the new binomial name Spissistilus festinus (Davis 1969).
Ceresa festina Say 1830
Membracis festina Say 1830:243
Stictocephala festina, Funkhouser, 1927:224-225
Spissistilus festinus, Metcalf and Wade, 1965:809-814
Distribution (Back to Top)
The native range of Spissistilus festinus is transcontinental, from Canada to Central America and West Indies (Wildermuth 1915). Spissistilus festinus is frequently found in the southern and southwestern United States. The species is less abundant in the northern United States and southern Canada (Osborn 1911, Wildermuth 1915, Caldwell 1949, Moellenbeck et al. 1993, Deitz and Wallace 2012).
Description and Identification (Back to Top)
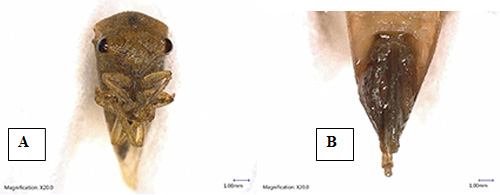
The adult Spissistilus festinus is light green and 6-7 mm long, with an elongated pronotum extending to the abdomen's tip (Wildermuth 1915). The dried specimen appeared a deep orange to straw color with suprahumerals and dorsal crest light red (Kopp and Yonke 1973). The pronotum has three corners, one at each shoulder and one at the apex. The male Spissistilus festinus can be differentiated from the females by observing a red tint on the dorsal surface of the pronotum. The female Spissistilus festinus is marginally bigger than the male (Wildermuth 1915) (Figure 2).
Figure 2. Adult male (A) and female (B) three-cornered alfalfa hopper, Spissistilus festinus Say, (Dorsal view). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
Spissistilus festinus is a hemimetabolous insect with three life stages, including eggs, nymphs, and adults. Eggs are white in color, and oblong in shape, with one end more expansive than the other, ranging from 0.9 to 1.3 mm long. The broader part of the egg is covered in papillae to secure the egg within the plant tissue (Wildermuth 1915). The female Spissistilus festinus inserts the eggs under the epidermis of the host plants in a slit. Spissistilus festinus oviposits near the base of the main stem early in the season in soybean (Glycine max L.) (Wildermuth 1915) and softer tissues such as terminals and nodes in the later of the season (Mitchell and Newsom 1984, Rice and Dress 1985). The number of eggs laid in each slit varies, averaging six eggs in each slit in soybean and 1-to 2 eggs per slit in alfalfa (Wildermuth 1915, Jordan 1952).
Figure 3. Adult three-cornered alfalfa hopper, Spissistilus festinus Say, (A, Ventral view) and female ovipositor (B). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
The number of nymphal instars (Figure 4) of Spissistilus festinus depends on nutrition and weather conditions. There are four to six nymphal instars, while five are more usual (Wildermuth 1915, Moore and Muller 1976, Deitz and Wallace 2012). The first and second instars are 1.6 mm and 2.1 mm in length, pale green or straw-colored with a series of dorsal spine-like protrusions. The dorsal spines grow and develop divergent lateral spurs that occur along the length of each spine after each successive nymphal stage. The third nymphal instars appear with wing pads and pronounced development of the pronotum. The third nymphal instars of Spissistilus festinus are darker yellow-brown with green markings and are about 2.9 mm in length. The fourth and fifth instars are similar in appearance and grow progressively greener with pronounced wing pads, dorsal spikes, and pronotum. The third through fifth instars are more mobile than the first and second instars (Wildermuth 1915). Nymphs can produce a globule from the abdomen as a defense mechanism and quickly move to the opposite side of the stem if disturbed. The adults have the same defensive manner as nymphs. Adults generally fly within 33 cm above the soil or the plant canopy (Johnson and Mueller 1989, 1990).
Figure 4. Fourth instar nymph (A) and different nymphal instars (B) of three-cornered alfalfa hopper, Spissistilus festinus Say, (Lateral view). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
Life History (Back to Top)
Depending on the favorable weather and food availability from the host plants, Spissistilus festinus can have multiple generations per year (Wildermuth 1915, Mitchell and Newsom 1984). The adult Spissistilus festinus undergoes a reproductive diapause in the winter (Newsom et al. 1983, Mitchell and Newsom 1984). However, Wildermuth (1915) found continued reproduction during mid-winters. Male Spissistilus festinus usually die soon after copulation but females live for 38.6 d post copulation (Mitchell and Newsom 1984). The sex ratio varies throughout the season (Mitchell and Newsom 1984, Newsom et al. 1983). However, some research shows that the Spissistilus festinus population consists of more males than females (Wildermuth 1915). A female Spissistilus festinus can produce more than 220 eggs during her reproductive period of 38 d (Mitchell and Newsom 1984). The incubation period ranges from 6 to 27 days (Meisch and Randolph 1965). Spissistilus festinus completes the first three instars in 3-5 days each and the last two instars in 4-8 days each (Wildermuth 1915, Jordan 1952, Meisch and Randolph 1965, Spurgeon and Mack 1990). Wildermuth (1915) reported that the total development time from egg hatching to adult was 69 days at 16°C and 32 days at 30°C.
Figure 5. Adult female and nymphs of three-cornered alfalfa hopper, Spissistilus festinus Say, (Lateral view). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
Hosts (Back to Top)
Spissistilus festinus has a wide range of feeding and reproductive hosts. However, plant species in the Fabaceae family are the most favorable as Spissistilus festinus infest those plants for their reproduction and development (Caviness and Miner 1962, Tugwell et al. 1972, Mitchell and Newsom 1984, Johnson et al. 1988). Spissistilus festinus was first recognized as a potential pest of tomato in 1888 in Georgia (Oemler 1888) and alfalfa in 1899 (Cockerell 1899). The host range of Spissistilus festinus includes tomato (Solanum lycopersicum L.), cowpeas (Vigna unguiculata L.), soybean (Glycine max L.), alfalfa (Medicago sativa L.), sugarcane (Saccharum offinarum L.), potato (Solanum tunerosum L.), cotton (Gossypium hirsutum L.), field pea (Pisum sativum L.), peanut (Arachis hypogaea L.), Bermuda grass (Cynodon dactylon L.), Johnson grass (Sorghum halepense L.), wheat (Triticum spp. L.), barley (Hordeum vulgare L.), oats (Avena sativa L.), bur clover (Medicago polymorpha L.), red clover (Trifolium pratense L.), sweet clover (Melilotus officinalis L.), sunflower (Helianthus spp. L.), mesquite (Prosopis glandulosa Torr.), Spanish clover (Acmispon americanus L.), subterranean clover (Trifolium subterraneum L.), crimson clover (Trifolium incarnatum L.), dandelion (Taraxacum officinale F. H. Wigg.), birdsfoot trefoil (Lotus corniculatus L.), common groundsel (Senecio vulgaris L.), field bindweed (Convolvulus arvensis L.), magnus peas (Pisum sativum L.), bell beans (Vicia faba L.), blando brome (Bromus hordeaceus L.), black medick (Medicago lupulina L.) (Wildermuth 1915, Van Zwaluwenburg 1926, Swezey 1937, Kopp and Yonke 1973, Anderson et al. 2002, Rahman et al. 2007). Spissistilus festinus was also found to overwinter and reproduce on a variety of alternative hosts, including weeds, pine (Pinus spp. L.), and vetch (Vicia spp. L.) (Wildermuth 1915, Osborn 1911, Mueller and Dumas 1987, Newsom et al. 1983).
Economic Importance (Back to Top)
Spissistilus festinus is a phloem feeder with piercing-sucking mouthparts. Both adults and nymphs show two distinct feeding behaviors, sporadic probing of stems and consumption of phloem sap (Anderson et al. 2002); and the formation of a continuous series of lateral punctures around the circumference of a stem, resulting in a girdle (gall-like growth) around the stem (Wildermuth 1915). Studies showed that insoluble salivary sheaths are left in the plant tissue after insect feeding, which disorganizes and disrupts the vascular bundles of the phloem (Smith 1933, Johnson et al. 1988). Mainly third, fourth, and fifth nymphal instars and adults can create stem girdles leading to lodging, stem breakage, and stand reduction (Caviness and Miner 1962, Meich and Randolph 1965, Bailey 1975, Tugwell et al. 1972, Moore and Muller 1976, Mueller and Jones 1983, Mitchel and Newsom 1984, Anderson et al. 2002). The nutrient flow becomes interrupted, and photosynthates accumulate in the area above the girdle leading to the breakage of the stem (Osborn 1911, Wildermuth 1915, Mitchel and Newsom 1984, Anderson et al. 2002). With the progression of plant age, the proportion of the feeding injury by Spissistilus festinus shifts from main stems to petioles (Rice and Dress 1985). The long-term adverse growth effects occur on the leaves and stem due to the temporary changes on the girdled stems. Nymphs of Spissistilus festinus were observed within 5 mm above the girdle and fed up to 7 days (Moellenbeck and Quisenberry 1991, Anderson et al. 2002). Heavy girdling reduces the forage quality, lowers carbohydrates and amino acids, and increases detergent fibers in alfalfa and soybean (Wilson and Quisenberry 1987, Mollenbeck and Quisenberry 1991). At the early vegetative stages of the host, Spissistilus festinus makes girdles mainly on the hypocotyl and first internode of the main stem which causes the thickness of the stem to increase and increased woodiness. Then hoppers shift to the petioles of leaves, small lateral branches, pods, pedicels, and peduncles, and even at the pod-filling stage (Mitchell and Newsom 1984). Recently, Spissistilus festinus was identified as a vector of Grapevine red blotch virus (GRBV) (Bahder et al. 2016). The infestation of GRBV delays maturity and reduces the accumulation of sugar in grapes. The disease also affects the production of secondary metabolites that impair grape quality in the form of color, flavor, and aroma (Blanco-Ulate et al., 2017). The grape growers and wine producers in the United States are very concerned about this vector-borne disease (Calvi 2011, Wallis and Sudarshana 2016). Nymphs and adults were observed in tomato and pepper fields infesting the seedlings and making girdles on the stems in South Florida (Khan and Seal personal communication 2021) (Figure 5, Figure 6, Figure 7). Adults and nymphs were also observed in nearby pepper and eggplant fields feeding on plants and making girdles on the stems (Adeleye and Garima, personal communication 2021) (Figure 9, Figure 10). Stems of some plants were completely broken because of the feeding injury (girdling) close to the root leading to the eventual death of the plant (Figure 11).
Figure 6. Nymphs of three-cornered alfalfa hopper, Spissistilus festinus Say, fed on tomato plant (Green circle) (A and B). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
Figure 7. Injured tomato seedlings (Girdle) (A and B) by nymphs and adults of three-cornered alfalfa hopper, Spissistilus festinus Say (Green circle). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
Figure 8. Injured tomato seedlings (A and B) (Girdle) by nymphs and adults of three-cornered alfalfa hopper, Spissistilus festinus Say, on tomato plant (Green circle). Photograph by Rafia A. Khan. Entomology and Nematology Department, University of Florida.
Figure 9. Injury (Girdle) by nymphs and adults of three-cornered alfalfa hopper, Spissistilus festinus Say, on pepper plant (Green circle). Photograph by Victoria Adeleye. Entomology and Nematology Department, University of Florida.
Figure 10. Feeding injury of three-cornered alfalfa hopper, Spissistilus festinus Say, on eggplant (Green circle). Photograph by Garima Garima. Entomology and Nematology Department, University of Florida.
Figure 11. Stem breakage caused by three-cornered alfalfa hopper, Spissistilus festinus Say, on pepper plant. Photograph by Victoria Adeleye. Entomology and Nematology Department, University of Florida.
Management (Back to Top)
Sampling
Spissistilus festinus females are found randomly distributed throughout the field in soybean and peanut Plant injury can be assessed within 10 m of field borders to adequately estimate whole field injury levels (Sparks and Boethel 1987, Rahman et al. 2007). The observation of the entire plant can provide an accurate assessment of the nymphal population but is time-consuming. Beat sheet sampling is less time-consuming but not ideal for detecting the younger, smaller nymphs in soybean (Spurgeon and Mueller 1991). The beat-net and vertical beat sheet techniques are alternatives to the beat sheet for sampling nymphs (Sparks and Boethel 1987, Dress and Rice 1985). The sweep net effectively assesses the adult population in soybean and peanut (Kogan and Herzog 1980, Rahman et al. 2007). Yellow sticky traps effectively monitor adults when placed 33 cm above the ground or plant canopy level (Johnson and Mueller 1988, Johnson and Mueller 1989).
Cultural control
Spissistilus festinus cannot survive nor reproduce on some cover cops like orchard grass (Dactylis glomerata L.), creeping red fescue (Festuca rubra L.), fawn tall fescue (Festuca arundinacea Schreb.), hard fescue (Festuca ovina L.), and California poppy (Eschscholzia californica Cham.). Cultivating these cover crops next to the crop field can reduce the migration of adult Spissistilus festinus to the crops (Kron and Sisterson 2020).
Biological control
Nickerson et al. (1977) found that the ant species of Solenopsis germinata Fabricius and Conomyrma insana Buckley were the most abundant and tended nymphs of Spissistilus festinus in Florida. He also reported the workers of ant species Conomyrma insane Buckley, C. flavopecta M.R. Smith, Iridomyrmex pruinosus Roger, and Pheidole morris Forel along with the previous two mentioned were observed to tend Spissistilus festinus in the soybean field. The entomopathogenic fungus Erynia delphacis (Hori) Humberwas found to be effective in controlling adult Spissistilus festinus in Alabama. The adults were found infected under laboratory conditions, where sporulation of fungi started through the intersegmental folds of the abdomen and the joint of frons and pronotum (Miller and Harper 1987). In Arkansas, Geocoris punctipes (Say) (Hemiptera: Geocoridae) and Nabis roseipennis (Reuter) (Hemiptera: Nabidae) were found to predate on Spissistilus festinus nymphs of 33% to 83% and 33% to 100%, respectively under laboratory conditions (Medal et al. 1997). Daigle et al. (1988) found Polynema spp. (Hymenoptera: Mymaridae) as the egg parasitoid of Spissistilus festinus. A strepsipteran parasite, Membracixenos jordani Pierce, was reported as a parasite of the Family Membracidae in America (Pierce 1952).
Chemical control
Insecticides of various modes of action (e. g. pyrethroids, carbamates, diamides) are an effective tool to reduce the Spissistilus festinus population. The third, fourth, and fifth nymphal instars cause most of the feeding injuries. Thus, management strategies need to reduce the population of those damaging stages (Beyer et al. 2017). However, the adult Spissistilus festinus can re-infest the field shortly after less effective chemical treatments (Sparks and Boethel 1987).
Acknowledgments (Back to Top)
Our special thanks goes to Dr. Susan Halbert and Dr. Mark J. Rothschild, Taxonomic Entomologist, Division of Plant Industry, Florida Department of Agriculture and Consumer Services, for their critical review to identify the species.
Selected References (Back to Top)
- Andersen, P. C., B. V. Brodbeck, and D. C. Herzog. 2002. Girdling‐induced nutrient accumulation in above ground tissue of peanuts and subsequent feeding by Spissistilus festinus, the three‐cornered alfalfa hopper. Entomologia experimentalis et applicata, 103(2):139-149.
- Bahder, B. W., F. G. Zalom, M. Jayanth, and M. R. Sudarshana. 2016. Phylogeny of geminivirus coat protein sequences and digital PCR aid in identifying Spissistilus festinus as a vector of grapevine red blotch-associated virus. Phytopathology 106(10):1223-1230.
- Bailey, J. C. 1975. Three-cornered alfalfa hoppers (Homoptera: Membracidae): effect of four population levels on soybeans. J. Kans. Entomol. Soc. 48:519-520.
- Beyer, B. A., R. Srinivasan, P. M. Roberts, and M. R. Abney. 2017. Biology and management of the three-cornered alfalfa hopper (Hemiptera: Membracidae) in alfalfa, soybean, and peanut. Journal of Integrated Pest Management 8(1):10,1-10.
- Blanco-Ulate, B., H. Hopfer, R. Figueroa-Balderas, Z. Ye, R. M. Rivero, A. Albacete, F. Pérez-Alfocea, R. Koyama, M. M. Anderson, R. J. Smith, and S. E. Ebeler. 2017. Red blotch disease alters grape berry development and metabolism by interfering with the transcriptional and hormonal regulation of ripening. Journal of experimental botany, 68(5):1225-1238.
- Caldwell, J. S. 1949. A generic revision of the treehoppers of the tribe Ceresini in America north of Mexico, based on a study of the male genitalia. Proceedings of the United States National Museum 98:491-521.
- Calvi, B. L. 2011. Effects of red-leaf disease on Cabernet Sauvignon at the Oakville experimental vineyard and mitigation by harvest delay and crop adjustment. M. S. thesis, University of California, Davis.
- Caviness, C. E., and F. D. Miner. 1962. Effects of Stand Reduction in Soybeans Simulating Three‐Cornered Alfalfa Hopper Injury 1. Agronomy Journal, 54(4):300-302.
- Cockerell, T. D. 1899. Some insect pests of the Salt River Valley and the remedies for them. College of Agriculture, University of Arizona (Tucson, AZ). Arizona Agricultural Experiment Station Bulletin 32:273-295.
- Daigle, C. J., A. N. Sparks Jr, D. J. Boethel, and P. L. Mitchell. 1988. Distribution of three-cornered alfalfa hopper (Homoptera: Membracidae) eggs in vegetative stage soybean. J. Econ Entomol 81(4):1057-1061.
- Davis, L. B. 1969. Biology of the Three-cornered Alfalfa Hopper, Spissistilus Festinus (Say), in Relation to Soybeans (Doctoral dissertation, Mississippi State University).
- Deitz, L. L. and M. S. Wallace. 2012. Richness of the Nearctic treehopper fauna (Hemiptera: Aetalionidae and Membracidae). Zootaxa, 3423(1):1-26.
- Drees, B. M., and M. E. Rice. 1985. The vertical beat sheet: a new device for sampling soybean insects. J. Econ. Entomol. 78(6):1507-1510.
- Kogan, M. and D. C. Herzog. 2012. Sampling methods in soybean entomology. Springer Science & Business Media.
- Kopp, D. D., and T. R. Yonke. 1973. The treehoppers of Missouri: Part 2. Subfamily Smiliinae; tribes Acutalini, Ceresini, and Polyglyptini (Homoptera: Membracidae). Journal of the Kansas Entomological Society 46(2):233-276.
- Kron, C. R., and M. S. Sisterson. 2020. Identification of nonhost cover crops of the three-cornered alfalfa hopper (Spissistilus festinus). American Journal of Enology and Viticulture, 71(3):175-180.
- Johnson, M. P., A. J. Mueller, W. M. Harris, and K. S. Kim. 1988. Histology of anomalous growth and adventitious roots associated with mainstem girdling by three-cornered alfalfa hopper, Spissistilus festinus (Say) (Homoptera: Membracidae) on soybean. Journal of Entomological Science 23(4):333-341.
- Johnson, M. P. and A. J. Mueller. 1989. Flight activity of the three-cornered alfalfa hopper (Homoptera: Membracidae) in soybean. J. Econ Entomol 82(4):1101-1105.
- Johnson, M. P. and A. J. Mueller. 1990. Flight and diel activity of the three-cornered alfalfa hopper (Homoptera: Membracidae). Environ Entomol 19(3):677-683.
- Jordan Jr, C. R. 1952. The biology and control of the three-cornered alfalfa hopper Spissistilus festinus (Say). Ph. D. dissertation Texas A&M University, College Station.
- Medal, J. C., A. J. Mueller, T. J. Kring, and E. E. Gbur Jr. 1997. Predation of Spissistilus festinus (Homoptera: Membracidae) nymphs by hemipteran predators in the presence of alternative prey. Fla Entomol. 80(4):451-456.
- Meisch, M. V., and N. M. Randolph. 1965. Life-history studies and rearing techniques for the three-cornered alfalfa hopper. J. Econ Entomol. 58(6):1057-1059.
- Moellenbeck, D. J., Quisenberry, S. S., and M. W. Alison Jr. 1993. Resistance of alfalfa cultivars to the three-cornered alfalfa hopper (Homoptera: Membracidae). Journal of Econ. Entomol. 86(2):614-620.
- Miller, M. K., and J. D. Harper. 1987. Occurrence of Erynia delphacis in the three-cornered alfalfa hopper, Spissistilus festinus (Homoptera: Membracidae). Journal of Invertebrate Pathology 50(1):81-83.
- Mitchell, P. L. and L. D. Newsom. 1984. Seasonal history of the three-cornered alfalfa hopper (Homoptera: Membracidae) in Louisiana. J. Econ. Entomol 77(4):906-914.
- Moellenbeck, D. J. and S. S. Quisenberry. 1991. Effects of nymphal populations of three-cornered Alfalfa Hopper (Homoptera: Membracidae) on ‘Florida 77’alfalfa plants. J. Econ. Entomol. 84(6):1889-1893.
- Moore, G. C., and A. J. Mueller. 1976. Biological observations of the three-cornered alfalfa hopper on soybean and three weed species. J. Econ. Entomol. 69(1):14-16.
- Mueller, A. J., and B. A. Dumas. 1987. Host plants of the three-cornered alfalfa hopper (Hemiptera: Homoptera: Membracidae). Environ. Entomol 16(2):513-518.
- Mueller, A. J., and J. W. Jones. 1983. Effects of main-stem girdling of early vegetative stages of soybean plants by threecornered alfalfa hoppers (Homoptera: Membracidae). Ibid. 76: 920-922.
- Newsom, L. D., P. Levin Mitchell, and N. N. Troxclair Jr. 1983. Overwintering of the three-cornered alfalfa hopper in Louisiana. J. Econ. Entomol. 76(6):1298-1302.
- Nickerson, J. C., C. R. Kay, L. L. Buschman, and W. H. Whitcomb. 1977. The presence of Spissistilus festinus as a factor affecting egg predation by ants in soybeans. Fla. Entomol. 60(3):193-199.
- Oemler, A. 1888. Extract from correspondence regarding a new tomato enemy in Georgia. In U. S. department of agriculture, division of entomology, Insect Life, 1(2):50.
- Osborn, H. 1911. Economic importance of Stictocephala. J. Econ. Entomol. 4(2):137-140.
- Preto, C. R., M. R. Sudarshana, and F. G. Zalom. 2018. Feeding and reproductive hosts of Spissistilus festinus (Say) (Hemiptera: Membracidae) found in Californian vineyards. J. Econ. Entomol. 111(6):2531-2535.
- Pierce, W. D. 1952. A new Strepsipterous parasite of Membracidae. Bu\. So. Cal. Acad. SCI. 51(1): 4-8.
- Rahman, K., W. C. Bridges Jr, J. W. Chapin, and J. S. Thomas. 2007. Threecornered alfalfa hopper (Hemiptera: Membracidae): seasonal occurrence, girdle distribution, and response to insecticide treatment on peanut in South Carolina. J. Econ. Entomol. 100(4):1229-1240.
- Rice, M. E. and B. M. Drees. 1985. Oviposition and girdling habits of the three-cornered alfalfa hopper (Homoptera: Membracidae) on preblooming soybeans. J. Econ. Entomol. 78(4):829-834.
- Smith, F. F. 1933. The nature of the sheath material in the feeding punctures produced by the potato leaf hopper and the three-cornered alfalfa hopper. Journal of Agricultural Research 47:475-485.
- Sparks Jr, A. N., and D. J. Boethel. 1987. Late-season damage to soybeans by three-cornered alfalfa hopper (Homoptera: Membracidae) adults and nymphs. Journal of Econ. Entomol.80(2):471-477.
- Spurgeon, D. W., and T. P. Mack. 1990. Development and survival of three-cornered alfalfa hopper (Homoptera: Membracidae) nymphs at constant temperatures. Environ. Entomol. 19(2):229-233.
- Spurgeon, D. W., and A. J. Mueller. 1991. Sampling methods and spatial distribution patterns for three-cornered alfalfa hopper nymphs (Homoptera: Membracidae) on soybean. J. Econ. Entomol. 84(3):1108-1116.
- Swezey, O. H., 1937. Notes on Potato Insects in Hawaii. In Proceedings of the Hawaiian Entomological Society 9(3):433-435.
- Tugwell, P., F. D. Miner, and D. E. Davis. 1972. Three-cornered alfalfa hopper infestations and soybean yield. J. Econ. Entomol 65(6):1731-1733.
- Van Zwaluwenburg, R. H., 1926. Insect enemies of sugarcane in western Mexico. J. Econ. Entomol. 19(4):664-669.
- Wallis, C. M. and M. R. Sudarshana. 2016. Effects of grapevine red blotch-associated virus (GRBaV) infection on foliar metabolism of grapevines. Canadian Journal of Plant Pathology 38(3):358-366.
- Wildermuth, V. L. 1915. Three-cornered alfalfa hopper. Journal of Agricultural Research 3:243-364.
- Wilson, H. K., and S. S. Quisenberry. 1987. Impact of feeding by three-cornered alfalfa hopper (Homoptera: Membracidae): greenhouse and field study. J. Econ. Entomol. 80(1):185-189.