common name: house fly
scientific name: Musca domestica Linnaeus (Insecta: Diptera: Muscidae)
Introduction - Distribution - Description and Life Cycle - Damage and Medical Importance - Economic Threshold - Management - Selected References
Introduction (Back to Top)
The house fly, Musca domestica Linnaeus, is a well-known cosmopolitan pest of both farm and home. This species is always found in association with humans or the activities of humans. It is the most common species found on hog and poultry farms, horse stables and ranches. Not only are house flies a nuisance, but they can also transport disease-causing organisms. Excessive fly populations are not only an irritant to farm workers but, when there are nearby human habitations, a public health problem could occur.
Figure 1. Adult house fly, Musca domestica Linnaeus. Photograph by Jim Kalisch, University of Nebraska-Lincoln.
Distribution
This common fly originated on the steppes of central Asia, but now occurs on all inhabited continents, in all climates from tropical to temperate, and in a variety of environments ranging from rural to urban. It is commonly associated with animal feces, but has adapted well to feeding on garbage, so it is abundant almost anywhere people live.
Life Cycle and Description (Back to Top)
The house fly has a complete metamorphosis with distinct egg, larval or maggot, pupal and adult stages. The house fly overwinters in either the larval or pupal stage under manure piles or in other protected locations. Warm summer conditions are generally optimum for the development of the house fly, and it can complete its life cycle in as little as seven to ten days. However, under suboptimal conditions the life cycle may require up to two months. As many as 10 to 12 generations may occur annually in temperate regions, while more than 20 generations may occur in subtropical and tropical regions.
Figure 2. Life cycle of the house fly, Musca domestica Linnaeus. Clockwise from left: eggs, larva, pupa, adult. Photograph by Jim Kalisch, University of Nebraska-Lincoln.
Egg: The white egg, about 1.2 mm in length, is laid singly but eggs are piled in small groups. Each female fly can lay up to 500 eggs in several batches of 75 to 150 eggs over a three to four day period. The number of eggs produced is a function of female size which, itself, is principally a result of larval nutrition. Maximum egg production occurs at intermediate temperatures, 25 to 30°C. Often, several flies will deposit their eggs in close proximity, leading to large masses of larvae and pupae. Eggs must remain moist or they will not hatch.
Figure 3. Adult and eggs of the house fly, Musca domestica Linnaeus. Photograph by Jerry F. Butler, University of Florida.
Larva: Early instar larvae are 3 to 9 mm long, typical creamy whitish in color, cylindrical but tapering toward the head. The head contains one pair of dark hooks. The posterior spiracles are slightly raised and the spiracular openings are sinuous slits which are completely surrounded by an oval black border. The legless maggot emerges from the egg in warm weather within eight to 20 hours. Maggots immediately begin feeding on and developing in the material in which the egg was laid.
The larva goes through three instars and a full-grown maggot, 7 to 12 mm long, has a greasy, cream-colored appearance. High-moisture manure favors the survival of the house fly larva. The optimal temperature for larval development is 35 to 38°C, though larval survival is greatest at 17 to 32°C. Larvae complete their development in four to 13 days at optimal temperatures, but require 14 to 30 days at temperatures of 12 to 17°C.
Nutrient-rich substrates such as animal manure provide an excellent developmental substrate. Very little manure is needed for larval development, and sand or soil containing small amounts of degraded manure allows for successful belowground development. When the maggot is full-grown, it can crawl up to 50 feet to a dry, cool place near breeding material and transform to the pupal stage.
Pupa: The pupal stage, about 8 mm long, is passed in a pupal case formed from the last larval skin which varies in color from yellow, red, brown, to black as the pupa ages. The shape of the pupa is quite different from the larva, being bluntly rounded at both ends. Pupae complete their development in two to six days at 32 to 37°C, but require 17 to 27 days at about 14°C). The emerging fly escapes from the pupal case through the use of an alternately swelling and shrinking sac, called the ptilinum, on the front of its head which it uses like a pneumatic hammer to break through the case.
Figure 4. Prepupa and sequence of puparia by age for the house fly, Musca domestica Linnaeus. Photograph by Jim Kalisch, University of Nebraska-Lincoln.
Adult: The house fly is 6 to 7 mm long, with the female usually larger than the male. The female can be distinguished from the male by the relatively wide space between the eyes (in males, the eyes almost touch). The head of the adult fly has reddish-eyes and sponging mouthparts. The thorax bears four narrow black stripes and there is a sharp upward bend in the fourth longitudinal wing vein. The abdomen is gray or yellowish with dark midline and irregular dark markings on the sides. The underside of the male is yellowish.
Figure 5. Adult house fly, Musca domestica Linnaeus. Photograph by Matt Aubuchon, University of Florida.
Figure 6. Lateral view of the head of an adult house fly, Musca domestica Linnaeus. Photograph by Matt Aubuchon, University of Florida.
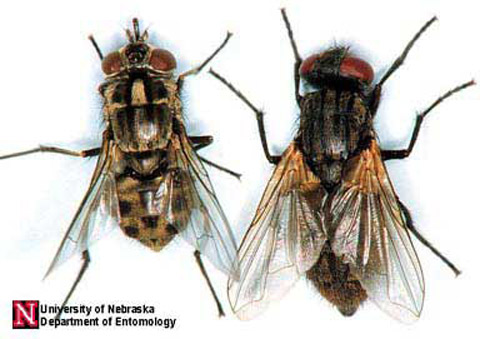
The house fly is often confused with the stable fly, Stomoxys calcitrans (Linnaeus), and the false stable fly, Muscina stabulans (Germar). All three are in the same family.
Figure 7. A dorsal comparison of adult stable fly, Stomoxys calcitrans (Linnaeus) (left), and house fly, Musca domestica Linnaeus (right). Photograph by Jim Kalisch, University of Nebraska-Lincoln.
Figure 8. A ventral comparison of adult stable fly, Stomoxys calcitrans (Linnaeus) (left), and house fly, Musca domestica Linnaeus (right). Photograph by Jim Kalisch, University of Nebraska-Lincoln.
Adults usually live 15 to 25 days, but may live up to two months. Without food, they survive only about two to three days. Longevity is enhanced by availability of suitable food, especially sugar. Access to animal manure does not lengthen adult life and they live longer at cooler temperatures. They require food before they will copulate, and copulation is completed in as few as two minutes or as long as 15 minutes. Oviposition commences four to 20 days after copulation. Female flies need access to suitable food (protein) to allow them to produce eggs, and manure alone is not adequate. The potential reproductive capacity of flies is tremendous, but fortunately can never be realized. Scientists have calculated that a pair of flies beginning reproduction in April may be progenitors, under optimal conditions and if all were to live, of 191,010,000,000,000,000,000 flies by August.
The flies are inactive at night, with ceilings, beams and overhead wires within buildings, trees, and shrubs, various kinds of outdoor wires, and grasses reported as overnight resting sites. In poultry ranches, the nighttime, outdoor aggregations of flies are found mainly in the branches, and shrubs, whereas almost all of the indoor populations generally aggregated in the ceiling area of poultry houses.
According to a study conducted in Texas, USA, breeding site suitability (in descending order), was horse manure, human excrement, cow manure, fermenting vegetable matter, and kitchen waste. However, another study found that structures containing swine, horse, sheep, cattle, and poultry varied in fly abundance, with swine facilities containing the most and poultry the least. Fruit and vegetable cull piles, partially incinerated garbage, and incompletely composted manure also are highly favored sites for breeding.
Damage and Medical Importance (Back to Top)
Flies commonly develop in large numbers in poultry manure under caged hens, and this is a serious problem requiring control. Although this fly species does not bite, the control of Musca domestica is vital to human health and comfort in many areas of the world. The most important damage related with this insect is the annoyance and the indirect damage produced by the potential transmission of pathogens (viruses, bacteria, fungi, protozoa, and nematodes) associated with this fly. Pathogenic organisms are picked up by flies from garbage, sewage and other sources of filth, and then transferred on their mouthparts, through their vomitus, feces and contaminated external body parts to human and animal food.
Of particular concern is the movement of flies from animal or human feces to food that will be eaten uncooked by humans. Also, when consumed by flies, some pathogens can be harbored in the mouthparts or alimentary canal for several days, and then be transmitted when flies defecate or regurgitate. In situations where plumbing is lacking, such as open latrines, serious health problems can develop, especially if there are outdoor food markets, hospitals, or slaughter houses nearby. Among the pathogens commonly transmitted by house flies are Salmonella, Shigella, Campylobacter, Escherichia, Enterococcus, Chlamydia, and many other species that cause illness. These flies are most commonly linked to outbreaks of diarrhea and shigellosis, but also are implicated in transmission of food poisoning, typhoid fever, dysentery, tuberculosis, anthrax, ophthalmia, and parasitic worms.
Economic Threshold (Back to Top)
The threshold density for determining when to control flies depends on the area where the control measures will be taken. In general, in homes the threshold is very low and control actions are taken with few flies. The complaint threshold density of the house fly at waste management sites may be 150 individuals per flypaper per 30 minutes.
House flies are monitored with baited traps, sticky ribbons, or spot cards on livestock facilities. Spot cards are 3-inch by 5-inch white index cards attached to fly resting surface. A minimum of five cards should be placed in each animal facility and left in place for seven days. A count of 100 or more fecal or vomit spots per card per week indicates a high level of fly activity and a need for control.
Tolerance of flies depends greatly on circumstances. In sensitive environments such as food preparation and packing facilities, restaurants, and hospitals, even small numbers of flies cannot be tolerated. In the context of livestock or poultry production, however, some flies are inevitable. Serious problems occur when cities or suburban development occur near poultry production facilities, as residents usually will not tolerate the large numbers of flies emanating from such facilities.
Management (Back to Top)
The more commonly used control measures for house flies are sanitation, use of traps, and insecticides, but in some instances integrated fly control has been implemented. The use of biological control in fly management is still at a relatively early stage.
Sanitation or cultural control. Good sanitation is the basic step in any fly management program. Food and materials on which the flies can lay eggs must be removed, destroyed as a breeding medium, or isolated from the egg-laying adult. Since the house fly can complete its life cycle in as little as seven days, removal of wet manure at least twice a week is necessary to break the breeding cycle. Wet straw should not be allowed to pile up in or near buildings. Since straw is one of the best fly breeding materials, it is not recommended as bedding. Spilled feed should not be allowed to accumulate. Ordinarily, fly control from 1 to 2 km around a municipality prevents house fly infestations.
Killing adult flies may reduce the infestation, but elimination of breeding areas is necessary for good management. Garbage cans and dumpsters should have tight-fitting lids and be cleaned regularly. Dry garbage and trash should be placed in plastic garbage bags and sealed up. All garbage receptacles should be located as far from building entrances as possible.
For control at waste disposal sites, refuse should be deposited onto the same area as inorganic wastes to deteriorate the capacity of breeding resources, or the disposed refuse should be covered with inorganic wastes (15 cm thickness).
Around homes and businesses, screening or covering of windows, doors or air doors, and trash containers proves useful in denying access of flies to breeding sites. Packaging household trash in plastic bags, and burying trash under at least 15 cm of soil and in sanitary landfills also helps to eliminate breeding.
In agricultural areas, manure can be scattered over fields so that it quickly dries and becomes unsuitable for egg and larval survival. Composting of manure can be effective if the compost is properly maintained, including regular turning. Manure can also be liquefied and stored in lagoons anaerobically, though at some point the solids need to be separated.
Traps. Fly traps may be useful in some fly control programs if enough traps are used, if they are placed correctly, and if they are used both indoors and outdoors. House flies are attracted to white surfaces and to baits that give off odors. Indoors, ultraviolet light traps collect the flies inside an inverted cone or kill them with an electrocuting grid. One trap should be placed for every 30 feet of wall inside buildings, but not placed over or within five feet of food preparation areas. Recommended placement areas outdoors include near building entrances, in alleyways, beneath trees, and around animal sleeping areas and manure piles. Openings to buildings should be tightly screened with standard window screen, thereby denying entrance to flies.
Traps can be baited with molasses, sugar, fruit or meat, and often are used in combination with a device that captures the attracted flies. The sex pheromone (Z)-9-tricosene also functions as an aggregation pheromone, and is called muscalure. Muscalure is formulated with sugar as a commercially-available fly bait for local population suppression, as well as an enhancement for population monitoring.
Ultraviolet light traps can be used to assess population levels, but also serve as a non-chemical control technique that can be used indoors in both agricultural and non-agricultural areas. They normally function by electrocuting flies that enter the trap, though those used in restaurants typically have a sticky panel. Flies do not orient to traps from a great distance, so several are normally needed for them to be effective. Placement should include within 4 to 8 m of entryways, and within 1.5 m of the floor, to take advantage of fly flight behavior. They should be operated continuously, although they are most effective when the room lights are off.
Biological control. With the increasing incidence of insecticide resistant house fly populations, rising costs of insecticides and a growing public concern about actual or potential problems associated with insecticides, interest in alternative house fly control strategies has increased.
Natural biological suppression of the house fly results primarily from the actions of certain chalcidoid wasps (Hymenoptera: Pteromalidae), of which many species have been associated with house fly around the world. Among the more important are Muscidifurax and Sphalangia spp. Ichneumonids and other parasitoids, as well as some predatory insects (especially histerids [Coleoptera: Histeridae] and staphylinids [Coleoptera: Staphylinidae]), also contribute to fly mortality, but under optimal fly breeding conditions the house fly quickly builds to high numbers. The more important in poultry facilities are the wasps Muscidifurax raptor and Sphalangia cameroni. Leaving a layer of old manure in the pits when manure is removed might enhance or stabilize the suppression of the house flies densities by parasitoids and predators.
Figure 9. House fly puparia, each with a hole from which a single wasp emerged after feeding on the pupa. Feeding occurs in the larval stage, and the wasp eventually emerges as an adult. Photograph by USDA.
Figure 10. Muscidifurax raptor wasp on a fly puparium. Once the female chooses a suitable puparium host, she lays a single egg in it. The egg hatches, and the wasp larva feeds on the fly pupa. Photograph by USDA.
Augmentative biological control (periodic release of parasitoids during winter and spring, and following manure removal) using insectary-reared parasitoids has been quite successful in some dairies, feedlots and poultry house situations. The species most often released for biological suppression in North America are Muscidifurax raptor, Muscidifurax raptorellus, Sphalangia endius, and Sphalangia nigroaenea. These different species function better under different conditions, some performing better under cooler or warmer conditions, others parasitizing flies near the surface or deeper in the pupation medium.
In North Carolina, tests showed that when house fly populations occur near the surface on the drier periphery of the manure, the conditions favor parasitism by Muscidifurax raptor. When the flies pupate at greater depths the conditions favor Sphalangia cameroni. In North Florida, releases conducted with Sphalangia endius showed that they could successfully parasitize pupae, both above and below the soil surface.
The larva of the black dump fly, Hydrotaea (=Ophyra) aenescens, is also regaining popularity as a biological control agent for controlling house flies on poultry farms without the use of pesticides. The adult black dump fly is similar in appearance to the adult house fly (Hogsette and Jacobs 2003).
Integrated fly control. Integrated fly control programs for caged-poultry houses are based on the following strategy:
- selective applications of insecticides against the adult,
- start insecticide control measures early in the spring before flies appear and repeat as frequently as needed through the warm months, and
- the manure is left undisturbed throughout the warm months when fly breeding may occur. The manure should be removed once very early in the spring before any flies appear.
Chemical control. When the house fly is a major pest in commercial egg production facilities, the control of this insect is by the application of adulticides or larvicides to directly or indirectly suppress adult densities. Residual wall sprays can be applied where the flies congregate. It is important to manage potential insecticide resistance by rotating formulations with different modes of action.
Outdoors, the control of flies includes the use of boric acid in the bottom of dumpsters, treatment of vertical walls adjacent to dumpsters and other breeding sites with microencapsulated or wettable powder formulation, and the use of fly baits near adult feeding sources.
Manure can also be treated with an insecticide, though this method is highly discouraged as it interferes with biological control of flies, often resulting in a rebound of the fly population. More commonly, insecticides (especially insect growth regulators) can be fed to livestock, and residual insecticide in the manure inhibits fly breeding. In animal facilities, insecticides are often applied to the favored resting places of adults, or bait stations established to poison adults with either solid or liquid formulations. Continuous exposure of flies to insecticides has led to development of insecticide resistance to many insecticides.
Indoors, the control of flies includes automatic misters, fly paper, electrocuting and baited traps that can be used in milk rooms and other areas of low fly numbers.
Selected References (Back to Top)
- Amano K. 1985. Breeding of the house fly, Musca domestica, (Diptera; Muscidae) in fresh dung of cattle fed on pasture grass. Applied Entomological Zoology 20: 143-150.
- Anderson JR, Poorbaugh JH. 1964. Observations on the ethology and ecology of various Diptera associated with Northern California poultry ranches. Journal of Medical Entomology 1: 131-147.
- Axtell RC. 1970. Integrated fly-control program for caged-poultry houses. Journal of Economic Entomology 63: 400-405.
- Barnard DR, Geden CJ. 1993. Influence of larval density and temperature in poultry manure on development of the house fly (Diptera: Muscidae). Environmental Entomology 22: 971-977.
- Bishoff FC, Dove WE, Parman DC. 1915. Notes on certain points of economic importance in the biology of the house fly. Journal of Economic Entomology 8: 54-71.
- Hedges SA. (ed.). 2004. The Mallis Handbook of Pest Control. 9th ed. GIE Media, Cleveland. 1396 pp.
- Hewitt CG. 1914. The House-Fly, its Structure, Habits, Development, Relation to Disease Control. University Press, Cambridge England.
- Hogsette JA, Jacobs RD, Miller RW. 1993. The sticky card: device for studying the distribution of adult house fly (Diptera: Muscidae) populations in closed poultry houses. Journal of Economic Entomology 86: 540-454.
- Hogsette JA, Jacobs RD. (2003). The black dump fly: A larval predator of house flies. EDIS. (no longer available online)
- Hogsette JA. 1996. Development of house flies (Diptera: Muscidae) in sand containing various amounts of manure solids and moisture. Journal of Economic Entomology 89: 940-945.
- Howard LO, Bishopp FC. 1925. The house fly and how to suppress it. USDA Farmers' Bulletin 1408. 18 pp.
- Imai C. 1985. A New Method to Control Houseflies, Musca domestica, at waste disposal sites. Research in Population Ecology 27: 111-123.
- Krafsur ES, Black IV WC, Church CJ, Barnes DA. 1985. Age structure and reproductive biology of a natural house fly (Diptera: Muscidae) population. Environmental Entomology 14: 159-164.
- Lord FT, Boston MD. 1904. Flies and tuberculosis. Boston Medical and Surgical Journal 151: 651-654.
- Lysyk TJ. 1991. Effects of temperature, food, and sucrose feeding on longevity of the house fly (Diptera: Muscidae). Environmental Entomology 20: 1176-1180.
- Lynsk TJ. 1993. Adult resting and larval development sites of stable flies and house flies (Diptera: Muscidae) on dairies in Alberta. Journal of Economic Entomology 86: 1746-1753.
- Kaufman PE, Scott JG, Rutz DA. 2001. Monitoring insecticide resistance in house flies from New York dairies. Pest Management Science 57: 514-521.
- Kaufman PE, Rutz DA. 2002. Susceptibility of house flies (Diptera: Muscidae) exposed to five commercial insecticides on painted plywood. Pest Management Science 58: 174-178.
- Kaufman PE, Rutz DA, Frisch S. 2005. Large sticky traps for capturing house flies, Musca domestica, and stable flies, Stomoxys calcitrans, in dairy calf greenhouse facilities. Journal of Dairy Science 88: 176-181.
- MacDonald RS, Surgeoner GA, Solomon KR. 1983. Development of resistance to permethrin and dichlorvos by the house fly (Diptera: Muscidae) following continuous and alternating insecticide use on four farms. Canadian Entomologist 115: 1555-1561.
- Merchant ME, Flanders RV, Williams RE. 1987. Seasonal abundance and parasitism of house fly (Diptera: Muscidae) pupae in enclosed, shallow-pit poultry houses in Indiana. Environmental Entomology 16: 716-721.
- Morgan PB, Weidhaas DE, Patterson RS. 1981. Programmed releases of Spalangia endius and Muscidifurax raptor (Hymenoptera: Pteromalidae) against estimated populations of Musca domestica (Diptera: Muscidae). Journal of Medical Entomology 18: 158-166.
- Ostrolenk M, Welch H. 1942. The common house fly (Musca domestica) as a source of pollution in food establishments. Food Research 7: 192-200.
- Ross EH. 1913 The Reduction of Domestic Flies. J.B. Lippincott Co. London 103 pp.
- Rutz DA, Axtell RC. 1981. House fly (Musca: Muscidae) control in broiler-breeder poultry houses by pupal parasites (Hymenoptera: Pteromalidae): indigenous parasite species and releases of Muscidifurax raptor. Environmental Entomology 10: 343-345.
- Rutz DA, Kaufman PE, Waldron JK. 2001. An Integrated Approach to Managing Fly Pests in Dairy Calf Greenhouses. 2000 NYS Livestock and Field Crops Project Reports Relating to IPM. NYS IPM Pub. #320. pp. 80-91.
- Savage EP, Scoof HF. 1955. The species composition of fly population at several types of problem sites in urban areas. Annals of the Entomological Society of America 48: 251-257.
- Seymour RC, Campbell JB. 1993. Predators and parasitoids of house flies and stable flies (Diptera: Muscidae) in cattle confinements in west central Nebraska. Environmental Entomology 22: 212-219.
- Scott JG, Alefantis TG, Kaufman PE, Rutz DA. 2000. Insecticide resistance in house flies from caged-layer poultry facilities. Pest Management Science 56: 1-7.
- Watson DW, Kaufman PE, Rutz DA, Glenister CS. 2001. Impact of the darkling beetle, Alphitobius diaperinus Panzer on the establishment of the predaceous beetle, Carcinops pumilio Erichson for Musca domestica control in caged-layer poultry houses. Biological Control 20: 8-15.
- West LS. 1951. The Housefly, its Natural History, Medical Importance, and Control. Comstock Publ Co. Ithaca, N.Y. 584 pp.