common name: oriental rat flea
scientific name: Xenopsylla cheopis (Rothschild, 1903) (Insecta:
Siphonaptera: Pulicidae)
Introduction - Synonomy - Identification - Distribution - Life Cycle and Biology - Medical Significance - Hosts - Economic Importance - Management - Selected References
Introduction (Back to Top)
The oriental rat flea, Xenopsylla cheopis (Rothschild, 1903), is a member of the family Pulicidae in the order Siphonaptera. Fleas are highly specialized, successful insect ectoparasites of birds and mammals (Marquardt et al. 2000). Of the nearly 2,500 species of fleas known from around the world (Triplehorn and Johnson 2005), few are as infamous as the oriental rat flea. This species is best known as one of the carriers of the plague bacterium Yersinia pestis, which is responsible for killing large numbers of people, including nearly a third of the human population of Europe during the Middle Ages, thus influencing the trajectory of human history (Fasulo 2004). In addition to its role as a vector of the plague bacterium, this species can vector other human pathogens such as Rickettsia typhi, the bacterium that causes flea-borne typhus, and at least two different tapeworms (Marquardt et al. 2000, Gage 2005).
Synonomy (Back to Top)
Xenopsylla cheopis (Rothschild, 1903) has various names in the literature. Synonyms include: Pulex cheopis N.C. Rothschild, 1903
Pulex murinus Tiraboschi, 1904
Alaopsylla murinus (Tiraboschi, 1904)
Alaopsylla philippinensis (Herzog, 1904)
Alaopsylla pachyruomyidis Glinkiewicz, 1907
Alaopsylla tripolitanus (Flumek, 1909)
(GBIF Secretariat 2019; ITIS Taxonomic Serial No. 189334).
Identification (Back to Top)
Fleas are easiest to identify in the adult stage, as eggs, larvae, and pupae often appear very similar among species. We therefore focus identification in this section on the adult stage.
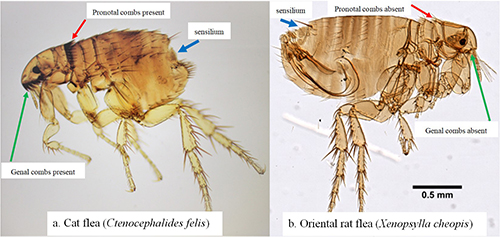
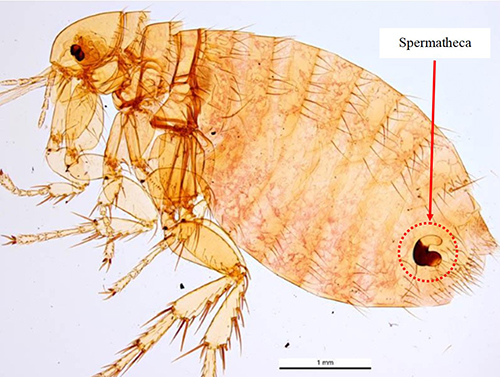
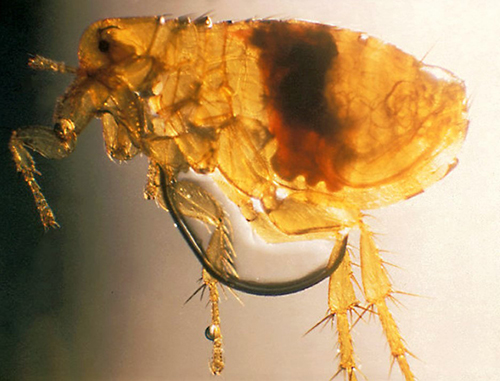
As is characteristic of fleas in general, a Xenopsylla cheopis adult is brown in color and laterally compressed (i.e., appearing flattened side-to-side). The adult generally ranges from 2-4 mm in length. The dorsal (upper) side of the head, thorax, and abdomen have setae, or bristles, that are directed posteriorly (toward the back) (Marquardt et al. 2000, Gage 2005) and the long legs bear numerous and noticeable spines to assist in movement on the host. The head is compact and helmet-like in appearance. This species is easily distinguished from many other fleas, such as the common cat flea, Ctenocephalides felis, and dog flea, Ctenocephalides canis, by the absence of genal and pronotal combs (distinct rows of bristles located below the eyes and behind the head, respectively). (Marquardt et al. 2000, Gage 2005) (Figure 1). An adult male and female flea are very similar in appearance, but a female flea possesses a conspicuous spermatheca (sperm storage vessel) in the posterior portion of the abdomen that can be seen when the flea is cleared and mounted for investigation (Figure 2).
Figure 1. Comparison images of Ctenocephalides felis and Xenopsylla cheopis. Note the presence of both the genal (cheek area below the eye; green arrow) and pronotal (behind the head; red arrow) combs in Ctenocephalides felis (a) and the characteristic absence of both combs in Xenopsylla cheopis (b). The sensory structure (sensilium) is present in both species and is described in the text. Photographs by Centers for Disease Control and Prevention (a) and Daniel Drew, Yale University (b).
Figure 2. Adult female Xenopsylla cheopis with red arrow pointing to the sperm storage vessel (spermatheca), which is encircled in red. Photograph by Ken Walker, Museum Victoria.
Distribution (Back to Top)
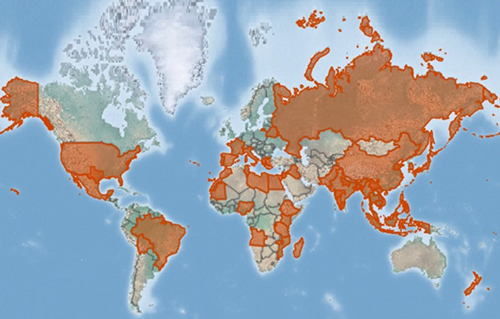
Xenopsylla cheopis is a cosmopolitan species, with a geographic distribution generally coinciding with its various hosts, including rats, gerbils, and other small mammals (Gage 2005) (Figure 3). Presumably, the only limiting factors for distribution of this species are temperature and humidity. Optimal temperatures are around 27°C with humidity at least 70% for successful egg laying (Trivedi 2003). Adults are quite tolerant to dry climates and moderate temperatures, though they may disappear during long, hot, dry spells and hence have environmental limitations on their overall distribution (Gage 2005).
Figure 3. Estimated distribution of Xenopsylla cheopis. Potential locations are shown in orange highlights and are based on database mining of location records. Map by the Centre for Agriculture and Bioscience International, United Kingdom.
Life Cycle and Biology (Back to Top)
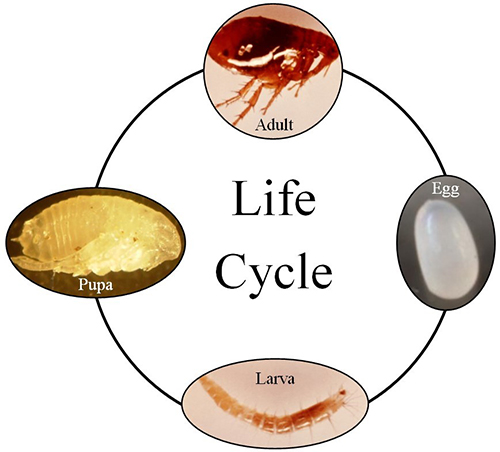
Fleas, including Xenopsylla cheopis, are holometabolous and have four distinct life stages: egg, larva, pupa, and adult (Figure 4).
Eggs: Flea eggs are ovoid in shape and have a smooth surface. Eggs are laid singly on either the host, or more commonly, in the nest and surrounding environment of the host. The egg stage is generally adapted to relatively high humidity, and females may produce up to six eggs daily and as many as 300-400 eggs during a lifetime (Gage 2005). Eggs are generally fully developed in approximately two weeks. The eggs are up to 0.05 mm in size, and likely contain nutrients from the mother (Trivedi 2003).
Larva: The larval stage hatches from the egg stage. Fleas have three instars of increasing size (ranging from 0.5-3 mm). Larval development takes about two weeks but has been recorded to take as long as 200 days in poor conditions (Trivedi 2003). The larvae live in nests and surrounding areas associated with their hosts (Marquardt et al. 2000, Trivedi 2003). Interestingly, Xenopsylla cheopis does not depend on blood-containing fecal matter as a food source like many other flea species, but rather can feed on detritus and even spilled cereal grains (Gage 2005).
Pupa: Larvae develop into pupae, and pupae range from 2-4 mm in length. Pupae are generally pale or light brown in color. Flea pupae are often encased in a silken cocoon adorned with small sand-size particles and dirt from the surrounding environment (Gage 2005). The length of time spent in the pupal stage appears to be directly related to ambient temperature and environmental cues and is suspected to range from 2-9 weeks. These cues include vibrations and chemical cues detected by the sensilium, a specialized sensory organ located on the posterior part of the abdomen (Figure 1) that may be used to detect ambient conditions and/or mating cues (Gage 2005, Greenwood and Holdich 2010); however, exact cues remain largely unknown.
Adult: Adult oriental rat fleas are obligate blood feeders, and both male and female adults feed on blood. They are telmophagic, meaning that they bite and then feed on blood that pools on the surface as opposed to siphoning it directly from beneath the skin. Blood is kept from coagulating through special enzymes in the saliva. In the flea foregut a muscular valve, the proventriculus, is responsible for breaking down the blood meal for digestion. The blocking of the proventriculus, a side effect of flea infection with the plague bacterium (see below), is thought to be a key factor related to this species’ preeminence as an effective spreader of the plague-causing bacterium, as this blockage can cause regurgitation into the host, as described below (Trivedi 2003). Adults can live for long periods of time, up to six weeks without a blood meal and well over 100 days with access to a blood meal (Gage 2005).
Figure 4. Generalized flea life cycle of showing all four stages of development. Adapted by the authors from photographs in the public domain.
Medical Significance (Back to Top)
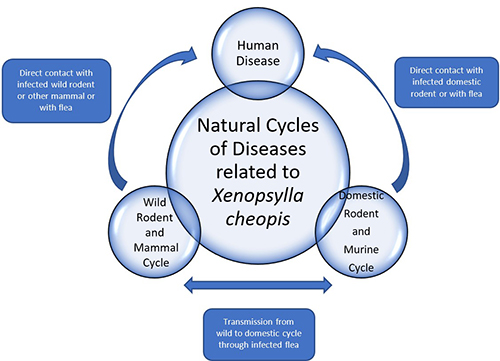
Several diseases can occur in humans because of their interactions with infected oriental rat fleas and/or their mammalian hosts (Figure 5). The oriental rat flea is best known from its association with plague-causing bacteria, which is suspected of causing mass military fatalities and decimating much of Europe during the Middle Ages (Fasulo 2004).
Figure 5. Generalized diagram of the disease cycles in which Xenopsylla cheopis is involved. Adapted by the authors from ideas in Marquardt (2000) and Gage (2005).
Plague, which is caused by the bacterium Yersinia pestis, has many potential forms in humans, such as bubonic (recognized by swollen lymph nodes), pneumonic (localized in the lungs), or septicemic (systemic blood infection). Yersinia pestis is known to block the proventriculus of the flea, causing it to regurgitate its blood meal, and hence release the bacterium into the host where it causes infection (Figure 6); however, Eisen et al. (2007) demonstrated that early Yersinia pestis transmission prior to blockage is still quite efficient. In the United States, the primary mammals of concern for harboring plague bacteria are prairie dogs (Cynomys spp.), black-footed ferrets (Mustela nigripes), rock squirrels (Otospermophilus variegatus) and wood rats (Neotoma spp.). In addition to Xenopsylla cheopis, important vectors of plague bacteria include the cat flea (Ctenocephalides felis) and rock squirrel flea (Diamanus montanus) (Cranshaw and Wilson, 2013).
Figure 6. Xenopsylla cheopis (Rothschild, 1903) with a blockage (seen as the dark, central mass) at the proventriculus (muscular valve at the entrance of the gut) after infection with the plague bacterium Yersinia pestis. Photograph by Dr. Pratt, CDC.
The oriental rat flea can also vector the bacterium Rickettsia typhi, which can cause the disease murine (flea-borne) typhus in humans. Rats and mice, as well as many other rodents, may serve as reservoirs of Rickettsia typhi. Humans can become infected when exposed to the rodents or to infected fleas (Figure 5). Though Rickettsia typhi can be transmitted by lice (Pediculus spp.), it is commonly found in cat fleasin Texas and California. However, Xenopsylla cheopisis the primary vector in other parts of the world (Afzal et al. 2017).
Xenopsylla cheopis can vector pathogens other than medically important bacteria (Gage 2005). Two tapeworms, namely the dwarf tapeworm, Hymenolepis nana, and the rodent tapeworm, Hymenolepis diminuta, can be spread to humans from rodents via the oriental rat flea. Additionally, Schwan et al. (2016), found a protozoan parasite (Trypanosoma lewisi) in Xenopsylla cheopis from sub-Saharan Mali, though the public health importance of this finding is not yet known.
Hosts (Back to Top)
The oriental rat flea can feed on the blood of many mammalian species but prefers rats (Rattus spp.) or other small mammals (e.g., many gerbil species) (Gage 2005). Domestic cats and dogs, as well as wild mammals, can also be hosts and consequently serve as source for pathogens transmitted by this flea. For example, cats are highly susceptible to plague bacteria and a common source of the bacteria for pet owners and veterinarians (CDC, 2020). Humans are accidental hosts for Xenopsylla cheopis (Figure 5).
Economic Importance (Back to Top)
Diseases like the plague caused by pathogens transmitted by Xenopsylla cheopis canhave strong negative economic impacts on humans. Fortunately, plague bacteria are easily treated with many antibiotics, and the cost of management can be relatively low. However, should the disease spread to the lungs or become septicemic, the cost of treatment can skyrocket to over $70,000 per patient. Murine typhus is similarly expensive to treat, with an average cost of nearly $17,000 per patient (Vohra et al. 2018). However, the greatest cost and concomitant economic impact of pathogens vectored by Xenopsylla cheopis is the loss of human life.
Management (Back to Top)
In addition to being found on hosts, fleas in multiple life stages can be found in the nests of hosts, complicating control of this species and leading to re-infestation of nearby structures if not managed effectively. Management of Xenopsylla cheopis and pathogens transmitted by the species involves a combination of host and vector reduction. Host reduction programs are designed to eliminate non-human hosts that may harbor the fleas. Rats and other small pest mammals are generally removed by trapping and poisoning. Applying insecticides to small mammal burrows or runways can also help to eliminate fleas (Gage 2005). Unfortunately, because of insecticide resistance in fleas, insecticides are becoming less effective at controlling Xenopsylla cheopis. In recent studies, most test populations were shown to be resistant to the most used pesticides (Miarinjara and Boyer 2016). Because controlling flea populations helps to control potential human pathogen infections, additional integrated pest management strategies to reduce host and flea populations, in addition to those already mentioned, are sorely needed.
Selected References (Back to Top)
- Afzal Z, Kallumadanda S, Wang F, Hemmige V, Musher D. 2017. Acute febrile illness and complications due to murine typhus, Texas, USA. Emerging Infectious Diseases 23: 1268-1273.
- CDC (Centers for Disease Control and Prevention). 2020. Plague: Information for veterinarians. https://tinyurl.com/y2a3v2bh (12 June 2020).
- Cranshaw W, Wilson R. (2013). Fleas and Plague. Colorado State University Extension, Fact Sheet 5.600. 3 p.
- Eisen RJ, Wilder AP, Bearden SW, Montenieri JA, Gage, KL. 2007. Early-phase transmission of Yersinia pestis by unblocked Xenopshylla cheopis (Siphonaptera: Pulicidae) is as efficient as transmission by blocked fleas. Journal of Medical Entomology 44: 678-682.
- Fasulo T. 2004. History and insects. In Resh, V. and Carde’, R., eds., Encyclopedia of Insects. Elsevier Academic Press, San Diego, CA. pp. 1077-1090.
- Gage KL. 2005. Fleas, the Siphonaptera. In Biology of Disease Vectors, WC Marquardt, Ed. Elsevier Academic Press, San Diego, CA. pp. 77-92.
- GBIF Secretariat. (2019). Xenopsyllacheopis(Rothschild, 1903). GBIF Backbone Taxonomy. Checklist dataset https://doi.org/10.15468/39omei. (21 May 2020).
- Greenwood MT, Holdich DM. 2010. A structural study of the sensilium of two species of bird flea, Ceratophyllus (Insecta: Siphonoptera). Journal of Zoology 187: 21-38.
- Hinnebusch BJ, Bland DM, Bosio CF, Jarrett CO. 2017. Comparative ability of Oropsylla montana and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLoS Neglected Tropical Diseases 11: e0005276. https://doi10.1371/journal.pntd.0005276.
- Marquardt WC, Demaree RS, Grieve RB. 2000. Fleas and flea-borne Diseases. In Parasitology and Vector Biology, 2nd ed. Harcourt Academic Press, San Diego, CA. pp. 569-582.
- Miarinjara A, Boyer S. 2016. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLoS Neglected Tropical Diseases 10: e0004414. https://doi:10.1371/journal.pntd.0004414.
- McKee CD, Osikowicz LM, Schwedhelm TR, Maes SE, Enscore RE, Gage KL, Kosoy MY. 2018. Acquisition of Bartonella elizabethae by experimentally exposed Oriental rat fleas (Xenopsylla cheopis; Siphonaptera, Pulicidae) and excretion of Bartonella DNA in Flea Feces. Journal of Medical Entomology 55: 1291-1298.
- Perez C, Hamouda D, Kamath B. 2018. Murine typhus: a life-threatening presentation of a case in Galveston, Texas. American Journal of Case Reports 19: 1503-1506.
- Roth JD. 2019. Sylvatic plague management and prairie dogs – a meta-analysis. Journal of Vector Ecology 44: e12323. https://doi.org/10.1111/jvec.12323.
- Schwan TG, Lopez JE, Safronetz D, Anderson JM, Fischer RJ, Maiga O, Sogoba N. 2016. Fleas and trypanosomes of peridomestic small mammals in sub-Saharan Mali. Parasites & Vectors 9: 541-548.
- Triplehorn CA, Johnson NF. 2005. Borror and DeLong's Introduction to the Study of Insects, 7th edition. Thomson Brooks/Cole, Blemont, CA. 864 p.
- Trivedi J. 2003. Xenopsylla cheopis. Animal Diversity Web, University of Michigan. https://animaldiversity.org/accounts/Xenopsylla_cheopis/ (09 June 2020).
- Vohra RF, Walker DH, Blanton LS. 2018. Analysis of health-care charges in murine typhus: Need for improved clinical recognition and diagnostics for acute disease. American Journal of Tropical Medicine and Hygiene 98: 1594-1598.
- Webb CT, Brooks CP, Gage KL, and Antolin MF. 2006. Classic flea-borne transmission does not drive plague epizootics in prairie dogs. Proceedings of the National Academy of Science 103: 6236-6241.
- Zhao F, Zhang T, Su J, Huang Z Wu A, Lin G. 2018. Genetic differentiation of the oriental rat flea, Xenopsylla cheopis, from two sympatric host species. Parasites & Vectors 11: 343-349.