common name: Pork Worm
scientific name: Trichinella spiralis (Paget, 1835) (Nematoda: Enoplea:Trichocephalida: Trichinellidae)
Introduction - Synonymy - Distribution - Description - Life Cycle and Biology - Hosts - Medical Importance - Economic Importance - Management - Selected References
Introduction (Back to Top)
Trichinella nematodes are parasites of animal intestines and cause the disease Trichinellosis or Trichinosis in humans, domestic pigs, horses, and many wild and feral animals; notably, these include bears, boars, foxes, cats, dogs, and rats. One such nematode, Trichinella spiralis, can cause a clinically significant, symptomatic infection in humans, which can progress to severe symptoms and death if not diagnosed and treated. These symptoms include nausea, vomiting, diarrhea, and abdominal pain, and usually begin within 2 days after eating undercooked meat from an infected animal. Trichinosis is diagnosed by a positive culture, antibody, or muscle biopsy test, and if untreated can cause fever, muscle pain, facial swelling, weakness and fatigue, headache, chills, and rash. In humans, many infections last weeks to months, until treated or overcome via immune response. Immune clearance of Trichinella spp. nematodes is more common for other Trichinella species than for Trichinella spiralis. In the case of a severe infection or heavier nematode load, neurological, motor, and cardiovascular trouble can develop and result in difficulty moving and breathing, and ultimately lead to death (CDC 2021; Cross & Lindquist 2017).
Trichinella spiralis is commonly known as “Pork worm” because infective larvae in undercooked infected pork is the most common cause of human infection. The infective larvae are encapsulated in cysts called “nurse cells” in the muscle tissue of the infected animal and are released upon digestion by pepsin and other digestive enzymes. Once inside the intestine, the nematodes can inhibit the host immune response while they mature, mate and produce more larvae, which then invade muscle tissue in the new host. This initial intestinal phase causes the various gastrointestinal associated symptoms of nausea, vomiting, diarrhea, and abdominal pain, while the invasion of skeletal and cardiac muscle by the new larvae results in the various other symptoms associated with Trichinosis.
Infections can be resolved by a course of antibiotics and antimicrobials. Human infection is always due to consumption of infected animals, and humans are considered a ‘dead-end’ host. Pigs are a common host as they are nearly ubiquitous world-wide, but European nations have had problems with infected foxes, boars, and other wild animals, as any carnivorous animal can potentially become infected with Trichinella. As a result of the extremely broad host range and host distribution across all continents, excluding Antarctica, Trichinella spp. are found globally, particularly where there is a larger concentration of humans and infected hosts which overlap (CDC 2021; Cross & Lindquist 2017).
Synonymy (Back to Top)
There are no reported synonyms for Trichinella spiralis (Paget, 1835). Several other members of the genus Trichinella are associated with human Trichinosis, though not all Trichinella nematodes are associated with human disease (CDC 2021).
Distribution (Back to Top)
Trichinella spiralis and related nematodes are globally distributed, occurring anywhere there are carnivorous or omnivorous animal populations, including throughout Europe, Asia, the Americas, and Africa, with lower occurrence in Israel and predominantly Muslim nations due to the reduced numbers of pig herds. Australia also has had limited exposure and few reported cases, compared with the rest of the world. Since its discovery in the mid-1800s, and the start of monitoring in 1866, Trichinella spiralis is known to have caused significant economic losses in Europe and the US. Monitoring in the European Union is extensive and costs upward of 600 million USD and has shown Trichinella to have maintained a strong presence in wild animal populations (Mitreva & Jasmer 2006; Murrell & Pozio 2011; Pozio 1998; Ribicich et al. 2007).
Description (Back to Top)
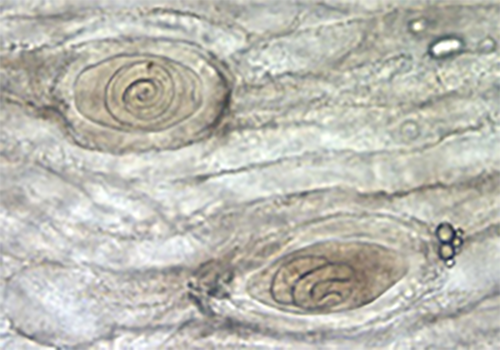
Trichinella spiralis is a relatively small nematode, with the fully grown adult measuring up to 1.4-1.6mm long. They are microscopic while in their larval stages and are thus very difficult to detect prior to encapsulation in host tissue. Following encapsulation, muscle tissue biopsies show a very characteristic nurse-parasite cell complex, which can be used to identify a Trichinella infection.
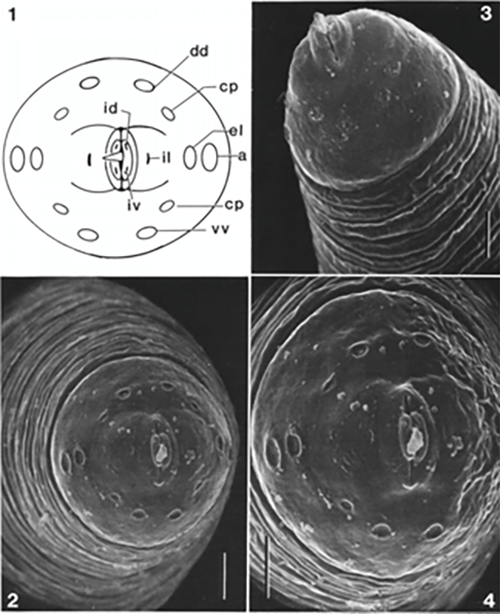
Males and female morphology differ greatly in appearance, though both have a very distinct parasitic-shaped piercing stylet, with twelve labial papillae, on two circles, and four cephalic papillae, and two amphids (Fig 1). Trichinella spiralis cuticle has ridges and creases along the longitudinal and horizontal axes, respectively (Fig 2). Unlike females, males have prominent bursae which play a role in mating. They have the appearance of a Y-shaped end, with multiple small swellings between the two prongs or extensions of the “Y.” Diagnosis of a Trichinella infection requires at minimum, an antibody test or tissue biopsy of a muscle from the patient. The pathologist will search for the characteristic ecapsulated cysts (Fig. 3) or signs of increased antibody titer against Trichinella spp. This is usually done if the patient can recall having consumed wild or potentially undercooked meats in the last week, prior to the onset of symptoms (Lichtenfels 1983; CDC 2021).
Figure 1. The stylet and papillae of Trichinella spiralis, showing multiple pairs of labial and cephalic papillae and a prominent and distinctive piercing-type stylet. Photograph from Lichtenfels et al. 1983.
Figure 2. The characteristic ridges and creases seen in the cuticle of Trichinella spiralis, under electron microscopy. Photograph from Lichtenfels et al. 1983.
Figure 3. Encapsulated larval cells in nurse cell-parasite cell complexes, as seen in the biopsy of bear muscle tissue. (Photograph by Centers for Disease Control and Prevention, https://www.cdc.gov/parasites/trichinellosis/diagnosis.html
Life Cycle and Biology (Back to Top)
The Trichinella spiralis life cycle begins with the consumption of infective encapsulated larvae. Encapsulated cysts from infected tissue, once ingested, are broken open during digestion to release infective larvae. Once within the host intestinal tract, the infective larvae embed into the gut mucosa and molt into their adult, sexually mature stage. Mated females can produce up to a thousand infective larvae per week. These larvae then invade striated muscle tissue, found in the skeletal and cardiac muscles, and encapsulate within the tissue, forming feeder nurse cells.
Trichinella spiralis have morphologically distinct males and females, the larvae of both sexes are present within encapsulated cysts in the tissues of infected animals (Lichtenfels 1983; Despommier 1998). Once infected tissue is consumed, both male and female larvae rapidly mature, by molting several times after embedding in the gastrointestinal epithelium (CDC 2021). Once sexually mature, they copulate, and the males are excreted (Ilic et al. 2012). The females remain in the gut for 1-2 weeks more, producing thousands of infective larvae which migrate through the blood and lymph of the host, to striated muscle tissues (Ilic et al. 2012). Once invasion is complete, larvae induce reorganization of striated muscle cells they have invaded. Chemicals secreted by the larvae cause the muscle cell to de-differentiate into a less committed cell. The process of nurse cell formation can be broken down into two stages, the de-differentiation of the muscle cell and the formation of the nurse cell from the more potent de-differentiated cell. During this induced de-differentiation, affected muscle cells lose characteristic striation and the internal myofibril organization is lost. Once they have de-differentiated, nearby satellite cells in the muscle tissue are activated. Without the necessary chemical signals from the infected muscle cells these cells are unable to mature into new muscle cells and instead become nurse cells which fuse with one another or with the central nurse cell. The nurse cells become the source of nutrients for the larvae and are highly proliferative (Ilic et al. 2012; Gottstein et al. 2009). As the nurse cells proliferate, dividing multiple times; the progeny fuse together to form a tightly joined protective wall, which encapsulates the larva, separating it from the host immune system, and allows it to mature and remain in the tissue for months to years (Ilic et al. 2012). The nurse cell-parasite complex is a unique structure which allows permanent remodeling of structure and genetic expression of infected muscle cells. Once in the cells, the larvae also attract small blood vessels for nutrients and to dispose of waste. This maintains a ‘clean’ environment which further improves their ability to evade host immune responses. (CDC 2021, Ilic et al. 2012; Despommier 1998; Ribicich et al. 2007).
Figure 4. The general lifecycle of the Trichinella spiralis nematode. The nematode can be maintained in domestic herds of agriculturally important animals, in wild populations, and can end in the human host after consumption of infected meats. Infective larvae and adults act much the same, within all hosts. Photograph by Centers for Disease Control and Prevention; https://www.cdc.gov/parasites/trichinellosis/biology.html.
Hosts (Back to Top)
Trichinella spiralis can infect any omnivorous or carnivorous animal; rats and pigs are the most common in areas with high human density, and wild animals in much of Europe and the US are also infected and have sustained Tricinella spp. in their populations (Gottstein et al. 2009). There is no definitive preferred host, though there are three ‘cycles’ consisting of wild and feral animal population, domestic animals, and dead-end human hosts. The consumption of the meat of an animal already infected by Trichinella is the only means of transmission of this nematode.
Medical Importance (Back to Top)
Parasitic infection with pork worm, or members of the Trichinella genus, is called Trichinosis and can be severe if not caught early (CDC 2021). Until the late 1900s, it was believed that only Trichinella spiralis could cause symptomatic infection in humans, but other species of the same genus can cause less severe or even asymptomatic infections as well. Early symptoms tend to be gastrointestinal as this reflects the initial embedding of infective larvae and maturation, and thus the first week after consumption of infected meat is marked by gastrointestinal distress, of varying severity (CDC 2021; Ribicich et al. 2007). Symptoms after the first week are associated with the movement of the nematode through the circulatory system, the invasion of muscle tissue, and the restructuring which occurs. Noticeable symptoms occur as a result of the high number of invading larvae, particularly in key muscle groups such as the fine motor muscles and cardiac muscle (Ribibich et al. 2007; Pozio 1998). Cardiovascular and motor trouble results from restructuring following muscle invasion.
Economic Importance (Back to Top)
There are limited costs noted in the US, due to minimal monitoring; however, the cost to human health is likely greater. Between 2011 and 2015, under 100 total cases were reported in the US. While more prevalent in the past, Trichinella spiralis cases have decreased significantly since the mid-20th century due to regulations prohibiting feeding raw meat to hogs and pork freezing practices. Now, more cases are associated with eating raw wild game meats than pork.
The European Union has instituted a heavy monitoring program to reduce and control outbreaks since 1866. Through this program, over 200 million animals are monitored annually, which can cost upwards of 600 million USD for monitoring of domestic animal herds alone. Further monitoring of wild populations, human health costs, and the costs of lost productivity from reduced animal growth, culling of infected herds, and other causes, are significantly greater than 1 billion USD, annually. (Ribicich et al. 2007; Murrell and Pozio 2011; Pozio 1998; Gottstein et al. 2009).
Management (Back to Top)
Management of Trichinella spiralis requires regular monitoring, such as that performed in the EU, and under the purview of the International Trichinella Reference Centre (ITRC). Once detected, all infected animals are killed and disposed of, and those herds are treated and monitored, as was the case for several outbreaks in Portugal, Spain, and the UK (Ribicich et al. 2007, Murrell and Pozio 2011). Freezing pork products and prohibiting the feeding of raw meat to hogs significantly decreases the risk of human infection. In humans, infection is treated with antiparasitic drugs such as albendazole, which target helminths specifically. If the infection is severe, with significant muscle invasion, steroids and other therapies are also used (CDC 2021).
Selected References (Back to Top)
- Cross, J., and H. Lindquist. 2017. Helminths, pp. 1763-1799. In Cohen, J., Powderly, W., Opal, S., Infectious Diseases. Elsevier. https://doi.org/10.1016/B978-0-7020-6285-8.00195-7.
- (CDC - DPDx - Trichinellosis) Cdc.gov. 2021. CDC - DPDx - Trichinellosis. (https://www.cdc.gov/dpdx/trichinellosis/index.html).
- Despommier, D.D. 1998. How does Trichinella spiralis make itself at home? Parasitology. 14: 318-323. https://doi.org/10.1016/S0169-4758(98)01287-3.
- Gottstein, B., Pozio, E., and K. Nockler. 2009. Epidemiology, diagnosis, treatment and control of Trichinellosis. American Society for Microbiology. 22: 127-145 https://doi.org/10.1128/CMR.00026-08.
- Ilic, N., A. Gruden-Movsesijan, and L. Sofronic-Milosavljevic. 2012. Trichinella spiralis: shaping the immune response. Immunologic Research. 52: 111-119. https://doi.org/10.1007/s12026-012-8287-5.
- Lichtenfels, J.R., Murrell, K.D., and P.A. Pilitt. 1983. Comparision of three supspecies of Trichinella spiralis by scanning electron microscopy. The Journal of Parasitology. 69:1131-1140. https://doi.org/10.2307/3280878.
- Mitreva, M. and D.P. Jasmer. 2006. Biology and genome of Trichinella spiralis. https://doi.org/10.1895/wormbook.1.124.1
- WormBook.,. 1-21. (https://digitalcommons.wustl.edu/open_access_pubs/3468/).
- Mitreva, M., Jasmer, D.P., Zarlenga, D.S., Wang, Z., Abubucker, S., Martin, J., Taylor, C.M., Yin, Y., Fulton, L., Minx, P., Yang, S., Warren, W.C., Fulton, R.S., Bhonagiri, V., Zhang, X., Hallsworth-Pepin, K., Clifton, S.W., McCarter, J.P., Appleton, J., Mardis, E.R. and R.K. Wilson. 2011. The draft genome of the parasitic nematode Trichinella spiralis. Nature Genetics. 43: 228-235. https://doi.org/10.1038/ng.769.
- Murrell, K.D. and E. Pozio. 2011. Worldwide occurrence and economic impact of human Trichinellosis, 1986-2009. Emerging Infectious Disease. 17: 2194-2202. https://doi.org/10.3201/eid1712.110896.
- Pozio.E. 1998. Trichinellosis in the European Union: epidemiology, ecology and economic impact. Parasitology Today. 14: 35-38. https://doi.org/10.1016/S0169-4758(97)01165-4.
- Ribicich, M., Gamble, H.R., Rosa, A., Sommerfelt, I., Marquez, A., Mira, G., Cardillo, N., Cattaneo, M.L., Falzoni, E., and A. Franco. 2007. Clinical, haematological, biochemical and economic impacts of Trichinella spiralis infection in pigs. Veterinary Parasitology. 147: 265-270. https://doi.org/10.1016/j.vetpar.2007.04.017.