common name: Guava root-knot nematode or pacara earpod root-knot nematode
scientific name: Meloidogyne enterolobii (Yang and Eisenback, 1983) (Nematoda: Chromadorea: Rhabditida:Tylenchina: Tylenchomorpha: Tylenchoidea: Meloidogynidae)
Introduction - Synonymy - Distribution - Description - Life cycle - Hosts - Symptoms - Management - Selected References
Introduction (Back to Top)
Meloidogyne enterolobii is an emerging tropical and subtropical pest. It was described by Yang and Eisenback (1983) but may have previously been misidentified as other root-knot nematode species. Guava root-knot nematode has the ability to overcome many resistant genes commonly deployed in crops and is particularly damaging, which makes it of particular concern for agricultural production.
Synonymy (Back to Top)
Meloidogyne mayaguensis (Rammah and Hirschmann, 1988)
Distribution (Back to Top)
Meloidogyne enterolobii was first described from the pacara earpod tree (Enterolobium contortisiliquum) in Hainan Island in China in 1983 (Yang and Eisenback, 1983), and is mainly found in tropical and subtropical areas including Africa (Benin, Burkina Faso, Congo, Kenya, Malawi, Mozambique, Niger, Nigeria, Senegal, South Africa, Togo); Asia (China, India, Thailand, Vietnam); Europe (Portugal, Switzerland); North America and the Caribbean (Costa Rica, Guadeloupe, Guatemala, Martinique, Mexico, Trinidad and Tobago, United States); and South America (Brazil, Venezuela (Castillo P and Castagnone-Sereno P, 2020). It has limited distribution but can be highly damaging. It was first reported in the United States in 2004 in Florida (Brito et al., 2004), and has been reported in most counties in Florida (Brito et al., 2008, 2010). It has since been reported in North Carolina (Ye et al., 2013), South Carolina (Overstreet et al., 2019) and Louisiana (Rutter et al., 2019).
Description (Back to Top)
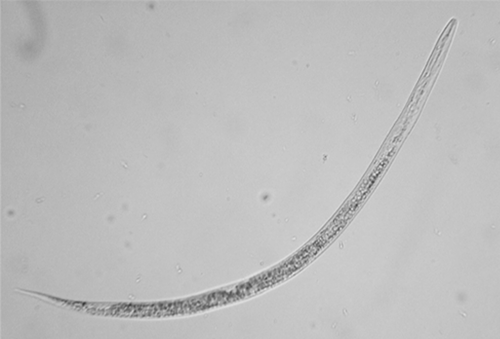
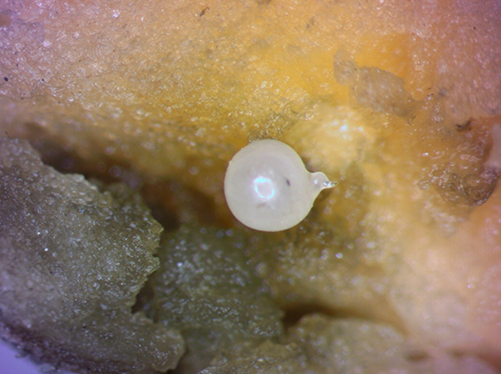
Morphologically, Meloidogyne enterolobii is very similar to other root-knot nematode species and requires training to differentiate nematodes morphologically. Second stage juveniles are translucent white, vermiform (worm-shaped), and tapered at both ends. They have a long, narrow tail and a delicate stylet (Figure 1). Females are white, pear-shaped with prominent necks variable in size (Figure 2). Males are translucent white, vermiform, and rounded at both ends (Figure 3). They are larger than juveniles with a more robust stylet and head framework. Morphological characteristics used to differentiate Meloidogyne enterolobii from other Meloidogyne species include the female perineal pattern (oval shape, coarse or smooth striae, moderately high to high arch, anus and vulva size and position) as well as the morphology of the head, tail, and stylet in various life stages (Yang and Eisenback, 1983). Identification based on molecular techniques can also be done and is often preferred as this requires only knowledge of common molecular techniques and no morphological expertise. Differentiation of Meloidogyne enterolobii from other common root-knot nematode species is based on the presence/absence and/or size of the amplicons in PCR reactions (Hu et al., 2011). The University of Florida Nematode Assay Lab performs this diagnosis by using molecular identification tools as do other professional diagnostic laboratories.
Figure 1. Second stage juvenile of Guava root-knot nematode Meloidogyne enterolobii at 400x magnification. Photograph from FINDME.
Figure 2. Guava root-knot nematode Meloidogyne enterolobii females are white and pear-shaped. Photograph by Will Rutter, USDA-ARS. Used by permission.
Figure 3. Meloidogyne male at 400x magnification. Males are worm-shaped with rounded heads and tails. Photograph by Zane Grabau, University of Florida.
Life cycle (Back to Top)
Meloidogyne enterolobii is very similar to other root-knot nematodes in respect to life cycle. The eggs hatch into 2nd-stage juveniles in the soil, which migrate through the soil in search for a susceptible host root. J2 stage is the only infective stage, and they invade the root tip and establish a permanent feeding site, from which the juvenile and adults feed. The root cells surrounding the feeding site enlarge and multiply, giving rise to a gall in which the juveniles are embedded. Nematodes keep developing into J3 and J4 stages, and eventually develop into globose females or vermiform males. Females produce eggs out of their body and eggs are deposited into a gelatinous matrix called “eggmass”. A single female can produce 500-1000 eggs. Mature males cease feeding and exit the roots. Males are not required for reproduction (mitotic reproduction).
Hosts (Back to Top)
As an emerging pest, Meloidogyne enterolobii host tests are still ongoing, but it can infect most horticultural, ornamental, and agronomic crops based on testing thus far (Table 1).
Meloidogyne enterolobii has a similar host preference to southern root-knot nematode Meloidogyne incognita, except that Meloidogyne enterolobii can reproduce on many plant cultivars carrying Meloidogyne resistance genes (Table 2). Thus far, sweetpotato production in the United States, guava production in Brazil, and horticultural production in the Caribbean are the industries that have been most affected by Meloidogyne enterolobii. Some crop species have been reported as poor or non-hosts for Meloidogyne enterolobii (Table 3), including grapefruit (Citrus paradisi), sour orange (Citrus aurantium), garlic (Allium sativum), coconut (Cocos nucifera), grape (Vitis vinifera), mango (Mangifera indica), mulberry (Morus alba), corn (Zea mays) and peanut (Arachis hypogaea) (Rodriguez et al., 2003; European and Mediterranean Plant Protection Organization, 2014; Freitas et al., 2017; Schwarz et al., 2020).
Table 1. List of common agronomic, horticultural and ornamental crops know to be good hosts to Meloidogyne enterolobii.
Common name |
Scientific name |
Reference |
Agronomic crops |
||
Cotton |
Gossypium hirsutum L. |
Ye et al., 2013 |
Soybean |
Glycine max |
Ye et al., 2013 |
sweet potato |
Ipomoea batatas |
Rutter et al., 2019 |
Tobacco |
Nicotiana tabacum |
Filho et al., 2016 |
Potato |
Solanum tuberosum |
Edward & Meleleki, 2013 |
Horticultural crops |
||
Thai basil |
Ocimum basilicum |
Gu et al., 2021 |
Bell pepper |
Capsicum annuum |
Assoumana et al., 2017 |
Cabbage |
Brassica oleracea |
Brito et al., 2007 |
Coffee |
Coffea |
Alves et al., 2009 |
Cucumber |
Cucumis sativus |
Kiewnick et al., 2008 |
Eggplant |
Solanum melongena |
Brito et al., 2007 |
Okra |
Abelmoschus esculentus |
Brito et al., 2007 |
Squash |
Cucurbita |
Brito et al., 2007 |
Tomato |
Solanum lycopersicum |
Kiewnick et al., 2008 |
Broccoli |
Brassica oleracea |
Brito et al., 2007 |
Banana |
Musa |
Silva et al., 2017 |
Guava |
Psidum guajava |
Gomes et al., 2008 |
Watermelon |
Citrullus lanatus |
Brito et al., 2007 |
Ornamental crops |
||
Angelonia |
Angelonia angustifolia |
Kaur et al., 2006 |
False daisy |
Eclipta prostrata |
Brito et al., 2008 |
Jamaican poinsettia |
Euphorbia punicea |
Han et al., 2012 |
Japanese blue berry |
Elaeocarpus decipiens |
Moore et al., 2020 |
Mulberryweed |
Fatoua villosa |
Brito et al., 2008 |
Poinsettia |
Poinsettia cyathophora |
Brito et al., 2008 |
Table 2. List of Meloidogyne resistance genes that Meloidogyne enterolobii is known to overcome. Crop listed under each resistance gene have cultivars that incorporate the given gene.
Resistance gene |
Crop common name |
Crop scientific name |
Reference |
Mi1 |
cotton |
Gossypium hirsutum |
Ye et al., 2013 |
Mi1 |
sweet potato |
Ipomoea batatas |
Rutter et al., 2019 |
Mi1 |
tomato |
Solanum lycopersicum |
Kiewnick et al., 2009 |
N |
pepper |
Capsicum annuum |
Kiewnick et al., 2009 |
Rk |
tobacco |
Nicotiana tabacum |
Ye et al., 2013 |
Table 3. List of common agronomic, horticultural, and ornamental crops known to be poor or non-hosts of Meloidogyne enterolobii.
Common name |
Scientific name |
Reference |
Agronomic crops |
||
corn |
Zea mays |
Rosa, J.M.O et al., 2011 |
peanut |
Arachis hypogaea |
Rodriguez et al., 2003 |
Horticultural crops |
||
garlic |
Allium sativum |
Rodriguez et al., 2003 |
grape |
Vitis |
Freitas et al., 2016 |
strawberry |
Fragaria ananassa |
Freitas et al., 2016 |
sour orange |
Citrus aurantium |
Freitas et al., 2016 |
passion fruit |
Passiflora edulis |
Freitas et al., 2016 |
Symptoms (Back to Top)
Symptoms caused by Meloidogyne enterolobii are similar to those caused by other root-knot nematode species, although on certain crops symptoms of Meloidogyne enterolobii infection are generally more severe than that of other root-knot nematode species. The typical symptom is severe galling (knotty root growths stimulated by Meloidogyne infection) of the root system (Figures 4 and 5). Meloidogyne enterolobii also infects tubers and storage roots, such as potatoes and sweetpotatoes (Figure 6). Infected tubers can be severely deformed with large galls on the tuber, the tuber can be cracked and dark on the surface, and white round females can be discovered under the tuber surface upon inspection with a dissecting scope. Aboveground symptoms include reduced stand, chlorosis (yellowing of plant foliage), stunting and loss of vigor (Figure 7). Damage symptoms on cultivars resistant to common Meloidogyne species are an indication that Meloidogyne enterolobii may be responsible for the damage as it can infect many resistant cultivars. As with other plant-parasitic nematodes, Meloidogyne enterolobii symptoms typically have a patchy field distribution (Figure 8) corresponding to varying nematode populations and environmental conditions.
Figure 4. Root galling induced by Meloidogyne enterolobii on a pepper carrying the N gene for resistance to most Meloidogyne species. Photograph by Zane Grabau, University of Florida.
Figure 5. Severe root galling induced by Meloidogyne enterolobii on tomato. Photograph by David Moreira Calix, University of Florida.
Figure 6. Meloidogyne enterolobii infected sweet potato with extensive galling and deep cracks. Photograph by Charles Overstreet, LSU. Used by permission.
Figure 7. Meloidogyne infected tomato exhibiting yellowing, stunting and wilting. Photograph by Chang Liu, University of Florida.
Figure 8. Patchy distribution of Meloidogyne symptoms (reduced stand, stunting of foliar) in a napa cabbage field. Photograph by Zane Grabau, University of Florida.
Figure 9. Reduced stand and stunting caused by Meloidogyne enterolobii in pepper production. Photograph by Johan Desaeger, University of Florida.
Management (Back to Top)
Management of Meloidogyne enterolobii relies largely on techniques that have been successful with other root-knot nematodes as relatively limited field research has been done with Meloidogyne enterolobii. Management options also vary by crop, particularly for chemical control. See UF/IFAS EDIS nematode management guides for information on specific crops including sweet potato, potato, cucurbits, tomatoes/peppers, cotton, and soybean among others.
Cultural: Extreme plant quarantine methods have been implemented to restrict dispersal of Meloidogyne enterolobii within the United States. After this pest was found in the North Carolina sweet potato growing belt in 2013, a number of states issued internal or external quarantines. These quarantines required planting material to be certified free of Meloidogyne enterolobii or restricted distribution of sweet potato produced from states confirmed to have this pest. Quarantine is a critical step in preventing this species spreading in a certain area. Using qualified nematode free seedlings and planting in clean fields even in areas where quarantines are not in place are important measures as they will prevent introduction of Meloidogyne enterolobii into the field.
Biological: Once Meloidogyne enterolobii is established in fields, it is generally not feasible to eradicate this pest and management relies on limiting the abundances of the nematode to alleviate crop damage. Crop rotation with poor- or non-hosts is a common and very successful method for managing other Meloidogyne species. The limited number of poor- or non-hosts for Meloidogyne enterolobii, particularly among high value crops, is the main factor that limits use of this practice. Biological control using fungal or bacterial agents may also be a useful tool for integrated pest management of Meloidogyne enterolobii. The fungus Trichoderma harzianum has shown value for managing Meloidogyne enterolobii on guava (Jindapunnapat et al, 2013), and the fungi Pochonia chlamydosporia and Purpureocillium lilacinum have had some efficacy in laboratory tests (Silva et al., 2017). Due to its ability to infect most known Meloidogyne resistant cultivars, use of resistant cultivars is currently unavailable to growers for managing this pest.
Chemical: There are both fumigant and non-fumigant nematicides registered for root-knot nematode management in Florida (Grabau, 2019). However, vegetables and ornamentals still largely rely on fumigation. Nematicides should be effective against Meloidogyne enterolobii, but more research on chemical control of this emerging pest is needed.
Acknowledgements: This document was produced as part of the FINDMe project with funding from USDA NIFA Specialty Crop Research Initiative (project #2019-51181-30018).
Selected References (Back to Top)
- Alves GCS, de Almeida EJ, and dos Santos JM. 2009. Reaction of Coffea spp. to Meloidogyne mayaguensis. Nematologia Brasileira 33: 248-251.
- Assoumana BT, Habash S, Ndiaye M, Van der Puije J, Sarr E, Adamou H, Grundler FMW, Elashry A. 2017. First report of the root-knot nematode Meloidogyne enterolobii parasitising sweet pepper (Capsicum annuum) in Niger. New Disease Reports 36: 18.
- Baojun Y, Eisenback JD. 1983. Meloidogyne enterolobii n. sp. (Meloidogynidae), a root-knot nematode parasitizing pacara ear pod tree in China. Journal of Nematology 15(3): 381-391.
- Brito JA, Powers TO, Mullin G., Inserra, RN, Dickson DW. 2004. Morphological and molecular characterization of Meloidogyne mayaguensis from Florida. Journal of Nematology 36(3): 232-240.
- Brito JA, Kaur R, Cetintas R, Stanley JD, Mendes M, McAvoy EJ, Powers TO, Dickson DW. 2008. Identification and isozyme characterisation of Meloidogyne spp. infecting horticultural and agronomic crops, and weed plants in Florida. Nematology 10(5): 757-766.
- Brito JA, Kaur R, Cetinas R, Stanley JD, Mendes ML, Powers TO, Dickson DW. 2010. Meloidogyne spp. infecting ornamental plants in Florida. Nematropica 40(1): 87-104.
- Brito JA, Smith T, Dickson DW. 2015. First report of Meloidogyne enterolobii infecting Artocarpus heterophyllus worldwide. Disease Notes 99(9): 1284-1285.
- Brito JA, Desaeger J, Dickson DW (2020). Reproduction of Meloidogyne enterolobii on selected root-knot nematode resistant sweetpotato (Ipomoea batatas) cultivars. Journal of Nematology. 52:1-6, DOI: https://doi.org/10.21307/jofnem-2020-063.
- Castagnone-Sereno P. 2012. Meloidognye enterolobii (= M. mayaguensis): profile of an emerging, highly pathogenic, root‐knot nematode species. Nematology 14(2): 133-138.
- Castillo P and Castagnone-Sereno P, 2020. Meloidogyne enterolobii (Pacara earpod tree root-knot nematode). Invasive Species Compendium. Wallingford, UK: CABI.
- Edward OM, Moleleki L. 2013. Detection of Meloidogyne enterolobii in potatoes in South Africa and phylogenetic analysis based on intergenic region and the mitochondrial DNA sequences. European Journal of Plant Pathology 136(1):1-5.
- Filho JVA, Machado JV, Dallagnol ACZ, Camargo LEA. 2016. Root-knot Nematodes (Meloidogyne spp.) parasitizing resistant tobacco cultivars in southern Brazil. Plant Disease 100(6): 1222-1231.
- Freitas VM, Silva JP, Gomes C, Castro JC, Correa V, Carneiro RDG. 2016. Host status of selected cultivated fruit crops to Meloidogyne enterolobii. European Journal of Plant Pathology 148(2): 307-319.
- Gomes CB, Couto MEO, Carneiro RMDG. 2008. Occurrence of Meloidogyne mayaguensis on guava and tabaco in south of Brazil. Nematologia Brasileira 32: 244-247.
- Gu M, Bui HX, Ye W and Desaeger J 2021. The first report of Meloidogyne enterolobii on Thai basil in Florida, United States. Plant Disease https://doi.org/10.1094/PDIS-02-21-0293-PDN
- Han H, Brito JA, Dickson DW. 2012. First report of Meloidogyne enterolobii infecting Euphorbia punicea in Florida. Plant Disease 96(11): 1706.
- Hu MX, Zhuo K, Liao JL. 2011. Multiplex PCR for the simultaneous identification and detection of Meloidogyne incognita, M. enterolobii, and M. javanica using DNA extracted directly from individual galls. Phytopathology 101(11): 1270-1277.
- Jindapunnapat K, Chinnasri B, Kwankuae S. 2013. Biological control of root-knot nematodes (Meloidogyne enterolobii) in guava by the fungus Trichoderma harzianum. Journal of Developments in Sustainable Agriculture 8 (2): 110-118.
- Kaur R, Brito JA, Dickson DW. 2006. First Report of Meloidogyne mayaguensis on Angelonia angustifolia. Plant disease 90(8): 1113.
- Kiewnick S, Karssen G, Brito JA, Oggenfuss M, Frey JE. 2008. First report of root-knot nematode Meloidogyne enterolobii on tomato and cucumber in Switzerland. Disease Notes 92(9): 1370.
- Kiewnick S. 2009. Effects of the Mi-1 and the N root-knot nematode-resistance gene on infection and reproduction of Meloidogyne enterolobii on tomato and pepper cultivars. Journal of Nematology 41(2): 134-139.
- Moore MR, Brito JA, Qiu S, Roberts CG. 2020. First report of Meloidogyne enterolobii infecting Japanese blue berry tree (Elaeocarpus decipiens) in Florida, USA. Journal of Nematology 52: e2020-05.
- Overstreet C, McGawley EC, Clark C, Rezende J, Smith T, Sistrunk M. 2018. Guava root knot nematode a potentially serious new pest in Louisiana. Pub 3670. Baton Rouge: Louisiana State University AgCenter.
- Rammah A, Hirschmann H. 1988. Meloidogyne mayaguensis n. sp. (Meloidogynidae), a root-knot nematode from Puerto Rico. Journal of Nematology 20(1): 58-69.
- Rodriguez M, Sanchez L, Rowe J. 2003. Host status of agriculturally important plant families to the root-knot nematode Meloidogyne mayaguensis in Cuba. 2003. Nematropica 33(2): 125-130.
- Rosa JMO, Westerich JN, Wilcken SRS. 2011. Reaction of maize hybrids and cultivars to Meloidogyne enterolobii and M. javanica. Nematologia Brasileira, 36(1): 9-14.
- Rutter WB, Skantar AM, Handoo ZA, Mueller JD, Aultman SP, Agudelo P. 2019. Meloidogyne enterolobii found infecting root-knot nematode resistant sweetpotato in South Carolina, United States. Plant disease 103(4): 775.
- Ye W, Koenning SR, Zhuo K, Liao J. 2013. First report of Meloidogyne enterolobii on cotton and soybean in North Carolina, USA. Plant Disease 97(9): 1262.
- Schwarz T, Li C, Ye W, Davis E. 2020. Distribution of Meloidogyne enterolobii in eastern North Carolina and comparison of four isolates. Plant Health Progress 21(2): 91-96.
- Silva S, Carneiro RMDG, Faria M, Souza DA, Monnerat RG, Lopez RB. 2017. Evaluation of Pochonia chlamydosporia and Purpureocillium lilacinum for suppression of Meloidogyne enterolobii on tomato and banana. Journal of Nematology, 49(1): 77-85.