common name: weevil parasitoid, pteromalid wasp, parasitoid wasp
scientific name: Catolaccus hunteri Crawford (Insecta: Hymenoptera: Pteromalidae)
Introduction - Synonymy - Distribution - Description and Life Cycle - Hosts - Economic Importance - Selected References
Introduction (Back to Top)
Catolaccus hunteri Crawford (Figure 1), is a natural enemy and ectoparasitoid of several insect species in the weevil family (Curculionidae), especially the pepper weevil (Anthonomus eugenii Cano), the boll weevil (Anthonomus grandis Boheman),and the Peruvian boll weevil (Anthonomus vestitus Boheman) (Cortez et al. 2004, Rodríguez-Leyva et al. 2007, Labbé et al. 2018, CABI, 2021). Each of these weevil species are important economic pests of their namesake host plant (Rodríguez-Leyva et al. 2000, Fernández et al. 2020). Catolaccus hunteri is considered one of the three most important parasitoid species to reduce pepper weevil populations (Rodríguez-Leyva et al. 2007).
Figure 1. Adult Catolaccus hunteri. Left: Female. Right: Male. Photograph by Garima Garima and Victoria Adeleye, Vegetable Entomology Lab, Tropical Research and Education Center, University of Florida.
Synonymy (Back to Top)
Former names of Catolaccus hunteri Crawford 1908 are:
Jaliscoa hunteri Crawford, 1908
Catolaccus townsendi Crawford, 1912
Zatropis hunteri Crawford, 1921
Heterolaccus hunteri Burks, 1954
Heterolaccus townsendi Burks, 1954
Pteromalus hunteri De Santis, 1979
Pteromalus townsendi De Santis, 1979
Catolaccus hunteri Burks, 1979 (Gibson 2013).
Distribution (Back to Top)
Literature suggests that the origin of Catolaccus hunteri is likely Mexico because it was first recorded on pepper weevil in Baja California Sur, Mexico (Aguilar and Sevin 2000). It has spread across North America (Canada, and United States) and South America (Colombia and Peru). In the United States, Catolaccus hunteri has been reported in Texas, South Carolina, Hawaii, and Florida (CABI 2021). In a survey conducted in ten locations in the Pacific and Atlantic coasts of Mexico, Catolaccus hunteri was collected at all sampled sites (Rodríguez-Leyva et al. 2007). Catolaccus hunteri, Triaspis eugenii (Wharton and Lopez Martinez, Hymenoptera: Braconidae) and Urosigalphus sp. represented 96% of all species collected from all sample sites and are the most important species in reducing pepper weevil populations. This is similar to results obtained by Chaires-Grijalva et al. (2021), where Catolaccus hunteri was the most abundant species found on four different pepper cultivars in Loma Bonita, Oaxaca, Mexico. Triaspis eugenii attacks pepper weevil eggs (Rodriguez-Leyva 2006).
In 1995, Catolaccus hunteri was recovered from samples of acerola weevil, Anthonomus macromalus Gyllenhal on acerola (Malpighia glabra L.) fruits in commercial sites located in Dade County, Florida (Hunsberger and Peña, 1997).
Figure 2. Larva of uninfested cowpea weevil, Callosobruchus maculatus used for rearing Catolaccus hunteri. Photograph by Garima Garima, Vegetable Entomology Lab, Tropical Research and Education Center.
Figure 3. Catolaccus hunteri emergence box. Rearing on chickpea seeds infested with cowpea weevil larvae. Photograph by Victoria Adeleye, Vegetable Entomology Lab, Tropical Research and Education Center.
Description and Life Cycle(Back to Top)
Oviposition period: Oviposition period of Catolaccus hunteri reared on cowpea weevil decreases as temperature increases. Oviposition period (±SE) is 51.10 ± 10.78, 30.20 ± 6.86, and 10.20 ± 1.85 days at 20.0°C, 25.0°C, and 30.0°C, respectively (Seal et al. 2002).
Eggs: Catolaccus hunteri eggs are translucent to white in color. Eggs are 448.42 ± 29.0 µm in length. Egg development period requires about 0.9 to 1.2 days (Seal et al. 2002). Under laboratory conditions, when reared on cowpea weevil larvae at 27 ± 1.0°C, the female eggs hatch in 1.17 ± 0.03 days and males take 1.18 ± 0.0 days to hatch (Rodríguez-Leyva et al. 2000). On or near pepper weevil larval host or cowpea weevil, female Catolaccus hunteri lay a single egg after using their ovipositor to pierce the host’s pericarp and can lay 10.93 ± 4.99 eggs per day during its maximum fecundity. This is when female adults are between 4 and 29 days old (Rodriguez-Leyva et al. 2000).
Larvae: Catolaccus hunteri larvae have 13 body segments. First instar larvae are about 0.5 - 0.7 mm in size and are translucent white. First instar larvae are more active than other larval stages and tend to show cannibalistic behavior at high densities. When Catolaccus hunteri is reared on cowpea weevil larvae at 27 ± 1.0°C, female larvae require 6.47 ± 0.73 days to pupate, and male larvae require 5.41 ± 0.64 days (Rodríguez-Leyva et al. 2000). Lowest larval developmental period was 4.6 ± 0.3 and 4.9 ± 0 days when reared on cowpea weevil and pepper weevil, respectively at 28°C (Seal et al. 2002).
Pupae: Newly developed pupae (0 - 36 h) are yellow in color, but the head, thorax and abdomen will gradually become black. The mean developmental time of Catolaccus hunteri from pupa to adult at 27 ± 1.0°C is 5.52 ± 0.58 days for females and 4.87 ± 0.21 days for males when reared on cowpea weevil (Rodríguez-Leyva et al. 2000). Pupal period varies depending on the host and temperature; the shortest pupal development period was recorded 4.5 ± 0.2 days on cowpea weevil and 4.9 ± 0 days on pepper weevil (Seal et al. 2002).
Adult: Adult Catolaccus hunteri are sexually dimorphic, meaning that males and females look different from one another. Adult females are larger (4 - 5 mm) than the males (2 - 3 mm) (Figure 1). Once the pupa becomes an adult, it makes an exit hole on the chickpea seed (Figure 4) and exits through it. Adult females usually have a pointed abdomen while the males have a translucent area in the front part of the abdomen (Figure 5) (Rodríguez-Leyva et al. 2000). The adult female’s ovipositor ranges from 1.42 to 2.27 mm in size (Gómez-Domínguez et al. 2012). Both male and female of Catolaccus hunteri have geniculate antennae (Figure 6). The antennae are shorter in males (1146.9 ± 22.1 µm) than females (1295.6 ± 15.6 µm) (Gómez-Domínguez et al. 2018). Catolaccus hunteri males live 58 days on cowpea weevil and 64 days on pepper weevil and females live 64 days on cowpea weevil and 69 days on pepper weevil at 28°C (Seal et al. 2002).
Catolaccus hunteri females reared on chickpea infested with larvae of the cowpea weevil laid 466.35 ± 280.39 eggs at 27°C, with a 1:1 female to male sex ratio (Rodriguez-Leyva et al. 2000). Adult females can lay 466.35 ± 280.39 eggs however only about 19% reach the adult stage due to cannibalistic nature of the 1st instar larva (Rodríguez-Leyva et al. 2000).
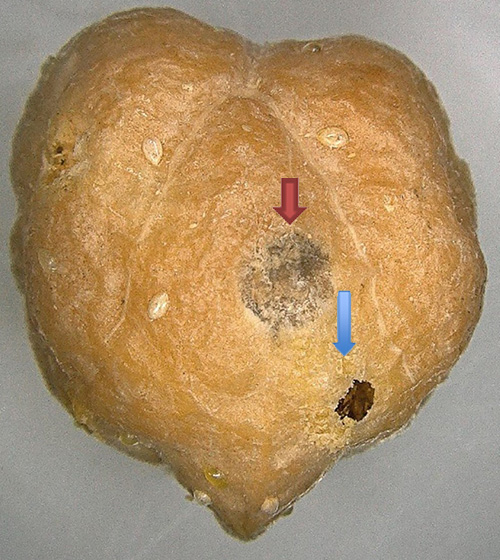
Figure 4. Exit hole of Catolaccus hunteri (blue arrow) and Callosobruchus maculatus adult (red arrow). Photograph by Garima Garima. Vegetable Entomology Lab, Tropical Research and Education Center.
Figure 5. A. Pointed abdomen of female Catolaccus hunteri (right) and translucent area on fore part of the male abdomen (left). B. Translucent area on fore part of the male abdomen (blue arrow). Photograph by Victoria Adeleye and Garima Garima, Vegetable Entomology Lab, Tropical Research and Education Center.
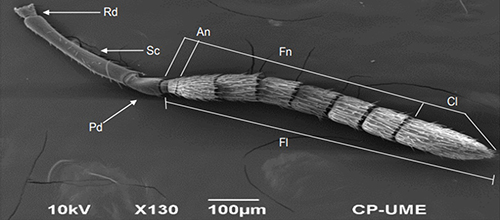
Figure 6. Geniculate antenna of Catolaccus hunteri male with radicle (Rd), scape (Sc), pedicel (Pd), and flagellum (Fl). Photograph by Nadia et al. 2018, used with permission.
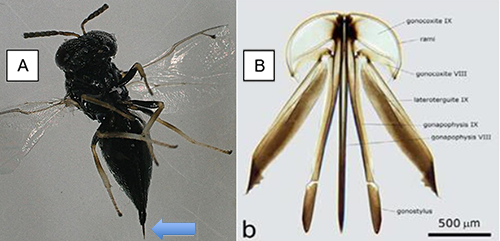
Figure 7: A. Female ovipositor (blue arrow). B. Structure of the female ovipositor. Photograph A by Victoria Adeleye, University of Florida, and B by Gómez-Domínguez et al. 2012, used with permission.
Hosts (Back to Top)
Catolaccus hunteri is known to parasitize boll weevil and pepper weevil larvae (Rodríguez-Leyva et al. 2007, Gómez-Domínguez et al. 2012, Murillo Hernandez et al. 2019) . It also parasitizes larvae of the acerola weevil. As with many other parasitoid wasps, Catolaccus hunteri inserts the tip of its ovipositor (Figure 7) into the host or prey to inject venom or puncture the body of the host and deposit an egg (Gómez-Domínguez et al. 2012). Common host plants of the prey of Catolaccus hunteri include pepper (Capsicum spp), and American black nightshade (Solanum americanum Mill) (Schuster 2007). It is rare to find Catolaccus hunteri in fruits that are over 2.5 cm diameter. This is because it is difficult to access third instar larvae which are usually deep inside larger fruits (Elmore et al. 1934, Rodriguez-Leyva et al. 2007). Therefore, it is recommended that the parasitoid is released early in the growing season when fruits are small (≤ 2.5 cm) (Schuster 2007). Catolaccus hunteri has been mass reared successfully under laboratory conditions on the non-host chickpea, Cicer arietinum L. infested with the larvae of cowpea weevil (Figure 2) (Seal et al. 2002, Vásquez et al. 2005, Rodríguez-Leyva et al. 2007, Authors personal observation, 2021, Garima 2021).
Economic Importance (Back to Top)
Catolaccus hunteri is the most common parasitoid of the pepper weevil in the United States and Mexico (Seal et al. 2002) and is also found attacking pepper weevil in Puerto Rico (Corria-Galindez et al. 2003). Catolaccus hunteri has been demonstrated as a successful biological control agent in the management of pepper weevil. For example, the release of Catolaccus hunteri combined with the removal of the only known alternate host in Florida, nightshade weeds, reduced pepper weevil infestations in three farms by 98%, 65%, and 80%, respectively (Brust et al. 2003). To date, Triaspis eugenii and Catolaccus hunteri have been evaluated as biological control agents for pepper weevil infestations.
In Florida, Catolaccus hunteri is the most abundant species of parasitoid for the biological control of pepper weevil but there has been little to no success for its use under field conditions (Rodríguez-Leyva et al. 2007, Schuster 2012). However, Schuster (2007) demonstrated that releasing 1,600 Catolaccus hunteri adults in a 0.2-hectare plot reduced the number of pepper weevil-infested bell peppers compared to a 0.2-hectare plot where Catolaccus hunteri was not released. Catolaccus hunteri exhibited similar effectiveness with Catolaccus grandis Burks (Hymenoptera: Pteromalidae) against the third instar larvae of the cotton boll weevil (Cortez et al. 2004). Mortality was observed in second instar and pupae of cotton boll weevil, and this was attributed to both parasitoid species (Cortez et al. 2004).
Garima (2021), evaluated the potential of Catolaccus hunteri in suppressing pepper weevil population under field conditions. There were no significant differences in pepper weevil infestation amongst treatments (pepper intercropped with nightshade and Catolaccus hunteri release, pepper intercropped with eggplant and Catolaccus hunteri release, pepper intercropped with cowpea and Catolaccus hunteri release, pepper sprayed with thiamethoxam, pepper with Catolaccus hunteri release, and pepper only). However, the number of pepper weevil adults observed, and infested jalapeño pepper fruits were lower on pepper plants intercropped with nightshade and plants where only Catolaccus hunteri was released compared to the rest of the treatments.
According to Vásquez et al. (2005), approximately 18,000 Catolaccus hunteri can be reared weekly under laboratory conditions (27C, 60% RH, and a photoperiod of 14L: 10D) on the cowpea weevil and chickpea with start-up costs of $1800 and weekly cost of $186 for human resources and supplies.
For supplies and human resources, it costs approximately 150$ (10$ chickpea, 1$ cotton roll, 2$ sugar, and 140$ human resources) weekly to produce 7-10 thousand Catolaccus hunteri adults under laboratory condition (27.5°C, 60-70% RH) in the vegetable entomology and nematology lab, Tropical Research and Education Center. Therefore, this organism may provide a viable option for biological control of the pepper weevil and other economically important weevil pests.
Catolaccus hunteri is susceptible to commonly used insecticides including thiamethoxam, beta-cyfluthrin, and oxamyl (Murillo Hernandez 2014, Authors personal observation, unpublished bioassay data). Catolaccus hunteri should not be released in any insecticide treated fields earlier than 10 days post application.
Selected References (Back to Top)
- Aguilar R, Servin R. 2000. First record of Catolaccus hunteri, a parasitoid of Anthonomus eugenii, in Baja California Sur, Mexico. Southwestern Entomologist. 25: 151-152.
- Berry PA. 1947. Anthonomus vesiitus and its natural enemies in Peru, and their importation into the United States. Journal of Economic Entomology. 40: 801-804.
- Brust GE, Mellinger C, Frantz G. 2003. An integrated approach to pepper weevil management in Florida using biological and cultural control. 2003 ESA Annual Meeting and Exhibition.
- CABI. 2021. Invasive species compendium: Catolaccus hunteri. Viewed on September 1st, 2021. Catolaccus hunteri (cabi.org).
- Chaires-Grijalva MP, Antonio-Luis MC, Palacios-Torres RE, Hernández-Hernández H, Castañeda-Vildozola A, Valenzuela-Escoboza FA, López-Martínez G. 2021. Nuevos registros de parasitoides del picudo del chile y su parasitismo natural en Loma Bonita, Oaxaca. Southwestern Entomologist. 45: 979-984.
- Correa-Galindez E, Armstrong A, Cruz C, Abreu E. 2003. New records of natural enemies of the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae), in Puerto Rico. The Journal of Agriculture of the University of Puerto Rico. 87:69-72.
- Cortez-Mondaca E, Barcenas-Ortega NM, Martinez-Carrillo JL, Leyva-Vázquez JL, Vargas-Camplis J, Rodríguez del-Bosque LA. 2004. Parasitism by Catolaccus grandis and Catolaccus hunteri (Hymenoptera: Pteromalidae) on the cotton boll weevil (Anthonomus grandis Boheman). Agrociencia. 38: 497-501.
- Elmore JC, Davis AC, Campbell RE. 1934. The pepper weevil.
- Fernández DC, VanLaerhoven SL, McCreary C, Labbé RM. 2020. An overview of the pepper weevil (Coleoptera: Curculionidae) as a pest of greenhouse peppers. Journal of Integrated Pest Management. 11:1-11.
- Garima G. 2021. Developing the method of rearing of Catolaccus hunteri Crawford, in the laboratory for mass production of the parasitoid. MSc. Thesis, Entomology and Nematology Department, University of Florida.
- Gibson GAP. 2013. Revision of the species of Jaliscoa Bouček within a review of the identity, relationships and membership of Jaliscoa, Catolaccus Thomson, Eurydinoteloides Girault, Lyrcus Walker and Trimeromicrus Gahan (Hymenoptera: Pteromalidae). Zootaxa. 3612: 1–85.
- Gomez-Dominguez NS, Lomeli-Flores JR, Rodríguez-Leyva E, Valdez-Carrasco JM, Bernal JS, Arzuffi R. 2018. Sense organs in antennae of Jaliscoa hunteri (Hymenoptera Pteromalidae). Bulletin of Insectology. 71: 103-111.
- Gómez-Domínguez NS, Refugio Lomeli-Flores J, Rodríguez-Leyva E, Valdez-Carrasco JM, Torres-Ruiz A. 2012. Ovipositor of Catolaccus hunteri Burks (Hymenoptera: Pteromalidae) and implications for its potential as a biological control agent of pepper weevil. Southwestern Entomologist. 37: 239–242. Gómez-Domínguez NS, Refugio Lomeli-Flores J, Rodríguez-Leyva E, Valdez-Carrasco JM, Torres-Ruiz.
- Hunsberger AGB, Peña JE. 1997. Catolaccus hunteri (Hymenoptera: Pteromalidae), a parasite of Anthonomus macromalus (Coleoptera: Curculionidae) in South Florida. Florida Entomologist. 80: 301-304.
- Labbé RM, Hilker R, Gagnier D, McCreary C, Gibson GAP, Fernández-Triana J, Mason PG, Gariepy TD. 2018. Host feeding by Jaliscoa hunteri on immature stages of pepper weevil. Southwestern Entomologist. 44: 775-778.
- Murillo-Hernandez JE. 2014. Toxicidad de insecticidas sobre Catolaccus hunteri Crawford (Hymenoptera: Pteromalidae) parasitoide de Anthonomus eugenii Cano (Coleoptera: Curculionidae). Maestro en Ciencias, Colegio de postgraduados, Campus Montecillo.
- Murillo-Hernandez JE, Garcia-Marinez Y, Rodriguez-Leyva E, Lomeli-Flores JR. 2019. Host feeding by Jaliscoa hunteri on immature stages of the pepper weevil. Southwestern Entomologist. 44: 775-778.
- Rodríguez-Leyva E. 2006. Life history of Triaspis eugenii Wharton and Lopez-Martinez (Hymenoptera: Braconidae) and evaluation of its potential for biological control of pepper weevil Anthonomus eugenii Cano (Coleoptera: Curculionidae). University of Florida.
- Rodríguez-Leyva E, Leyva JL, Gómez V, Barcenas NM, Elzen GW. 2000. Biology of Catolaccus hunteri (Hymenoptera: Pteromalidae), a parasitoid of pepper weevil and boll weevil (Coleoptera: Curculionidae). Annals of the Entomological Society of America. 93: 862-868.
- Rodríguez-Leyva E, Stansly PA, Schuster DJ, Bravo-Mosqueda E. 2007. Diversity and distribution of parasitoids of Anthonomus eugenii (Coleoptera: Curculionidae) from Mexico and prospects for biological control. Florida Entomologist. 90: 693-702.
- Schuster DJ. 2007. Suppression of Anthonomus eugenii (Coleoptera: Curculionidae) pepper fruit infestation with releases of Catolaccus hunteri (Hymenoptera: Pteromalidae). Biocontrol, Science, and Technology. 17: 345-351.
- Schuster DJ. 2012. Response of Catolaccus hunteri (Hymenoptera: Pteromalidae) to colored sticky traps in the laboratory. Florida Entomologist. 95: 501-502.
- Seal DR, Stansly PA, Schuster DJ. 2002. Influence of temperature and host on life history parameters of Catolaccus hunteri (Hymenoptera: Pteromalidae). Environmental Entomology. 31: 354-360.
- Vásquez E, Dean D, Schuster D, Van Etten P. 2005. A laboratory method for rearing Catolaccus hunteri (Hymenoptera: Pteromalidae), a parasitoid of the pepper weevil (Coleoptera: Curculionidae). Florida Entomologist. 88: 191-194.