common name: Asian bush mosquito, Asian rock pool mosquito
scientific name: Aedes japonicus japonicus (Theobald, 1901) (Insecta: Diptera: Culicidae)
Introduction - Synonymy - Distribution - Life Cycle - Medical Importance - Surveillance and Management - Selected References
Introduction (Back to Top)
Aedes japonicus japonicus (Theobald, 1901; Figure 1), commonly known as the Asian bush or rock pool mosquito, is an invasive container-inhabiting mosquito native to Korea, Japan, Taiwan, Southern China, and Russia that has become established in parts of Europe, Canada, and in most of the eastern United States (Tanaka et al. 1979, Kaufman and Fonseca 2014). Successful establishment beyond its native distribution is partly due to this species’ adaptability to a broad range of environmental conditions. Aedes japonicus adjusts well to human environments and is more tolerant of cooler, temperate climates than other invasive container-inhabiting mosquito species, enabling it to successfully invade a diverse array of habitats (Versteirt et al. 2009).
Figure 1. Adult female Aedes japonicus (Theobald) with golden stripes on scutum (dorsal area of thorax). Photograph by Sean McCann, Simon Fraser University.
While Aedes japonicus has not been implicated as an important arthropod-borne virus (arbovirus) vector (Kampen and Werner 2014), its documented human blood feeding and propensity to live in close association with humans warrants surveillance and management activities, especially in areas at risk of new introductions (Fonseca and Kaufman 2010, Jansen et al. 2018). Aedes japonicus’ pathway of introduction is unknown in many instances, but transportation in used tires (Figure 2) via international trade is a likely candidate. This introduction pathway was confirmed in New Zealand, where larval Aedes japonicus were found in shipments of tires arriving from Japan (Laird et al.1994). International tire trade was also the introduction pathway for another important invasive mosquito species, the Asian tiger mosquito, Aedes albopictus (Skuse), making this the suspected source of Aedes japonicus in many other countries, where adults are often detected in tire yards (Hawley et al. 1987, Versteirt et al. 2009).
Figure 2. In addition to serving as a potential method of transport, tires that are discarded and left in the environment collect water, providing developmental habitat for container-inhabiting species like Aedes japonicas (Theobald). Photograph by Amy Hallock, University of Florida.
Synonymy (Back to Top)
Ochlerotatus japonicus japonicus (Reinert 2000)
Hulecoeteomyia japonica japonica (Reinert et al. 2006)
Distribution (Back to Top)
In its native range of Korea, Japan, Taiwan, Southern China, and Russia, Aedes japonicus japonicus is one of four subspecies and two sibling species that are difficult to differentiate between using only morphological characteristics: Aedes japonicus japonicus, Aedes japonicus shintienensis Tsai & Lien, 1950, Aedes japonicus amamiensis Tanaka, Mizusawa & Saugstad, 1979, Aedes japonicus yaeyamensis Tanaka, Mizusawa & Saugstad, 1979, and Aedes koreicus (Tanaka et al. 1979). For reasons not quite understood, Aedes japonicus japonicus has become an aggressive invasive species in North America and Europe, establishing itself well beyond its native range in eastern Asia within the past two decades. Aedes japonicus was first documented in the United States in 1998, where it was detected in Connecticut and shortly thereafter, New York and New Jersey (Peyton et al. 1999, Andreadis et al. 2001). Aedes japonicus rapidly expanded its North American range, becoming established throughout much of eastern United States and parts of Canada in approximately fifteen years. Population genetic analyses of this species show that its spread throughout the Northeast was made possible through multiple introductions (Fonseca et al. 2001).
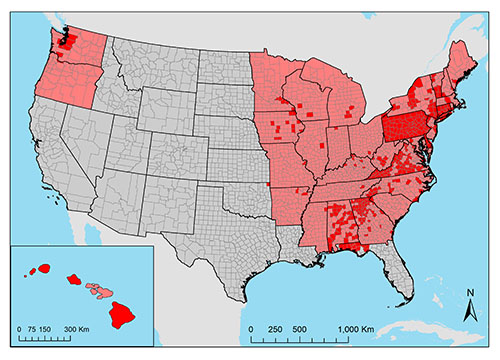
Aedes japonicus has also been found in all states east of the Mississippi river, in addition to midwestern states (Iowa, Missouri, and Minnesota), south central states (Arkansas and Oklahoma), the Pacific northwest (Oregon and Washington), and Hawaii (Figure 3; Peyton et al. 1999, Bevins 2007, Kaufman and Fonseca 2014, Riles et al. 2017, Bradt et al. 2018). Aedes japonicus in Florida was first documented in Okaloosa County in 2012. It has since been recorded in other counties in the Panhandle of Florida, including Bay, Leon, Santa Rosa, and Walton counties (Riles et al. 2017).
Figure 3. The current introduced range of Aedes japonicus (Theobald) in the United States. State-level occurrences reported in Kaufman and Fonseca 2014 are shown in pink. Documented county-level occurrences are shown in red (Peyton et al. 1999, Andreadis et al. 2001, Scott 2003, Kaufman et al. 2005, Butler et al. 2006, Bevins 2007, Andreadis et al. 2010, Johnson et al. 2010, Kaufman et al. 2012, Winchester and Kapan 2013, Egizi et al. 2016, Riles et al. 2017, Bradt et al. 2018, GMCA 2018, Hutchinson 2018, McKenzie et al. 2019, Peach et al. 2019, VAAFM 2019, Sames et al. 2020, Smith et al. 2020). Map created in ArcMap (version 10.6) by Catherine Lippi, University of Florida.
Life Cycle (Back to Top)
Aedes japonicus is a cold-tolerant, multivoltine holometabolous insect, undergoing complete metamorphosis with egg, larval, pupal, and adult life stages within multiple generations a year (Andreadis et al. 2001, Byrd et al. 2019).
Eggs: After ingesting a blood meal, female mosquitoes lay their eggs in water-holding containers, above the water line. Eggs are small, approximately 0.5 mm in length, matte black in color, and cigar shaped (Figure 4; Haddow et al. 2009). Aedes japonicus produces eggs that are resistant to both desiccation and sub-zero temperatures, enabling the eggs to overwinter in diapause and resume development once environmental conditions become favorable (Scott 2003, Andreadis and Wolfe 2010, Medlock et al. 2015). The developmental time of eggs is temperature-dependent, although temperatures above 30°C have been shown to inhibit development and emergence in laboratory conditions (Scott 2003).
Figure 4. The eggs of Aedes mosquitoes are small (0.5 mm in length), black, and cigar shaped. Photograph by CDC Public Health Image Library.
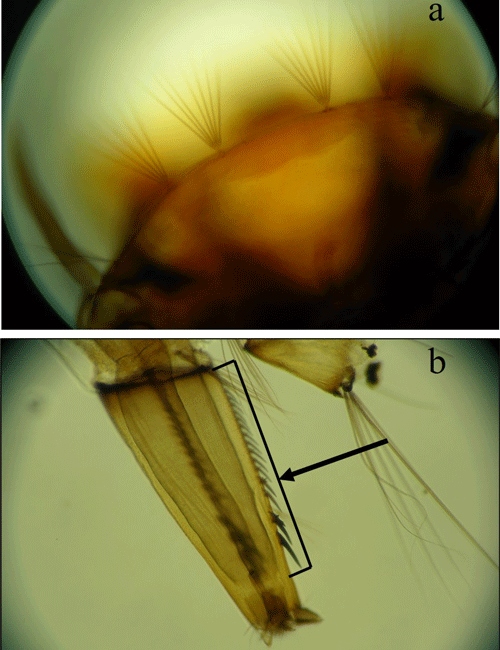
Larvae: Mosquito larvae hatch from eggs and actively feed on organic matter in the water. Larval Aedes japonicus are distinguished from other mosquito species found in North America by their branched setae along the front of the head capsule arranged in a straight row, highly spiculated (spiny) anal saddle, and pecten that runs almost the entire length of the siphon with teeth that are larger and further apart closer to the siphon’s tip (Figure 5).
Figure 5. (a) Branched setae along front margin of larval Aedes japonicus (Theobald) head arranged in straight row. (b) Pecten along almost entire length of the larval Aedes japonicus siphon, which is made up of teeth that are larger and further apart closer to the siphon’s tip. Photographs by George O’Meara, University of Florida.
Compared to other Aedes larvae, Aedes japonicus can typically be found earlier in the year when temperatures are cooler. In the northeastern United States, the period of active larval development takes place from March to November (Andreadis et al. 2001). Provided there are no sustained freezes (i.e., temperatures in aquatic habitat falling below 10°C), larvae are capable of overwintering in both their native and introduced ranges. Larvae can successfully complete their development in a variety of water-filled natural (i.e., rock pools) and man-made containers, the quality of which vary considerably in light exposure and organic matter content (Andreadis et al. 2001, Juliano and Lounibos 2005, Lorenz et al. 2013, Kampen and Werner 2014). Since its establishment in the Northeast, the occurrence of immature Aedes japonicus has increased relative to two native species, Aedes triseriatus found in discarded tires and Aedes atropalpus found in rock holes, but mechanisms underlying these changes are unclear (Andreadis and Wolfe 2010).
Pupae: Aedes japonicus larvae enter the pupal stage after their larval development is complete. Although they do not feed in this life stage, pupae are mobile, readily swimming to avoid predation. Pupae take approximately two days to develop or longer at cooler temperatures, after which adult mosquitoes emerge.
Adults: In the northeastern United States, adult Aedes japonicus mosquitoes are active from May to November (Andreadis et al. 2001). Adult mosquitoes of both sexes feed on sugars and plant nectars as a food source, while only females take blood meals to fully develop eggs. Adult femaleshave been documented primarily taking blood meals from mammals, including humans, but are also known to bite birds (Molaei et al. 2009, Schönenberger et al. 2016). As container-inhabiting mosquitoes, adult females lay their eggs in natural and artificial water-holding containers. Tree holes, rock pools, and bamboo plants are examples of natural containers. Artificial containers include debris, used tires, flower pots, rainwater collection basins, and concrete structures.
As an adult, Aedes japonicus is medium-sized (Burkett-Cadena 2013). Female mosquitoes are typically larger in size and lack the plumose (feather-like) antennae found on males. Aedes japonicus can be easily distinguished from many native mosquito species in North America by the presence of golden stripes on its scutum, black and white scales on the sides of the thorax, and black and white banding on the legs (Figure 6).
Figure 6. In addition to the golden stripes on the scutum, black and white scales on the sides of the thorax, and black and white banding on the legs, are useful in distinguishing Aedes japonicas (Theobald) from native mosquito species in North America. Photograph by Lyle J. Buss, University of Florida.
Similar in appearance to other invasive but now established Aedes container-inhabiting mosquitoes in the United States, Aedes japonicus is diagnosable by the golden lyre-like pattern on its scutum. In contrast, the yellow fever mosquito, Aedes aegypti (Linnaeus), has a silver lyre on the scutum, the Asian tiger mosquito, Aedes albopictus, has a silver line down the middle of the scutum (Figure 7).
Figure 7. Although similar in appearance to other container-inhabiting species found in Florida, the gold lyre-like pattern on the scutum easily distinguishes Aedes japonicus (Theobald) (left), from Aedes aegypti (center), and Aedes albopictus (right). Photographs by Lyle J. Buss, University of Florida.
Medical Importance (Back to Top)
Aedes japonicus can transmit Japanese encephalitis virus (JEV; an important arbovirus in its native range) both horizontally and vertically in the laboratory (Takashima and Rosen 1989). However, its role in the JEV transmission cycle is unknown (Kampen and Werner 2013). Under laboratory conditions, Aedes japonicus is also a competent vector of a range of other arboviruses including chikungunya, dengue virus, eastern equine encephalitis, Saint Louis encephalitis, Rift Valley fever, West Nile, and Zika (Turell et al. 2001 and 2013, Sardelis et al. 2002 and 2003, Schaffner et al. 2011, Kaufman and Fonseca 2014, Jansen et al. 2018).
In North America and Europe, wild-caught Aedes japonicus mosquitoes have tested positive for West Nile and La Crosse viruses. As a result, Aedes japonicus has been suggested as a potential bridge vector of West Nile and La Crosse viruses to humans (Turell et al. 2005, Versteirt et al. 2009). However, more field-collected evidence is needed to determine the role it plays within these transmission cycles (Schaffner et al. 2013).
Regardless, Aedes japonicus has been noted as a mosquito pest in areas where it has become established. Although not particularly aggressive, its willingness to take human blood meals (Figure 8) and its reproductive success in areas of human habitation make this species a potential nuisance (Schaffner et al. 2003).
Figure 8. An adult female Aedes japonicus (Theobald) taking a blood meal from a human. Photograph by James Gathany, CDC Public Health Image Library.
For more information on medically important mosquitoes in Florida, visit the UF/IFAS Florida Medical Entomology Laboratory website at https://fmel.ifas.ufl.edu/
For more information on mosquito-borne pathogens in Florida, visit the UF/IFAS Extension website at http://edis.ifas.ufl.edu/topic_mosquito-borne_diseases
Surveillance and Management (Back to Top)
As an invasive species, Aedes japonicus is often first detected through routine surveillance activities for other mosquitoes of public health importance (Connelly 2004). While common surveillance traps like the Centers for Disease Control and Prevention (CDC) miniature light trap, New Jersey light trap, and BG-Sentinel trap adult mosquitoes can be used to detect adults, they are likely to underestimate Aedes japonicus population size (Kaufman and Fonseca 2014). Though, baiting a CDC miniature light trap with an octenol lure and carbon dioxide has been shown to significantly increase Aedes japonicus adult catch rates (Anderson et al. 2012).
Larval surveillance is likely to be more successful than adult surveillance and can be conducted using dark-colored, five-gallon buckets containing leaf infusion (Johnson et al. 2010, Kaufman et al. 2012). An integrated pest management strategy that includes source reduction (Figure 9) and selective use of chemical controls like larvicides can be used to reduce container-inhabiting mosquito populations. Eliminating larval mosquito habitats around homes, particularly water-holding artificial containers (Figure 10), reduces the number of mosquitoes potentially produced around households. Supplementing container elimination with targeted larvicidal treatments of developmental habitats has been demonstrated to successfully reduce the abundance of Aedes japonicus (Ibañez-Justicia et al. 2018).
Figure 9. Emptying containers of standing water helps eliminate developmental sites for Aedes japonicus and other container-inhabiting mosquitoes. Photograph by Lauren Bishop, CDC Public Health Image Library.
Figure 10. Artificial containers that collect water, such as an uncovered garbage can, may provide developmental habitats for container-inhabiting Aedes mosquitoes like Aedes japonicus. Photograph by Catherine Lippi, University of Florida.
Personal protective measures can reduce the risk of exposure to mosquito bites. These personal protective measures include applying an insect repellant and wearing long pants and a long-sleeved shirt when engaging in outdoor activities as well as limiting outdoor activity during times of peak mosquito activity (Rutledge and Day 2002).
For more information on managing mosquitoes and personal protective measures, please visit the UF/IFAS Extension Mosquito Control website at http://edis.ifas.ufl.edu/topic_mosquito_control
Selected References (Back to Top)
- Anderson JF, McKnight S, Ferrandino FJ. 2012. Aedes japonicus japonicus and associated woodland species attracted to Centers for Disease Control and Prevention miniature light traps baited with carbon dioxide and the Traptech® Mosquito Lure. Journal of the American Mosquito Control Association 28:184-91. DOI: 10.2987/12-6260R.1.
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. 2001. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. Journal of Medical Entomology 38: 774-779. DOI: 10.1603/0022-2585-38.6.774.
- Andreadis TG, Wolfe RJ. 2010. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the northeastern United States. Journal of Medical Entomology 47: 43-52. DOI: 10.1093/jmedent/47.1.43.
- Bevins SN. 2007. Establishment and abundance of a recently introduced mosquito species Ochlerotatus japonicus (Diptera: Culicidae) in the Southern Appalachians, USA. Journal of Medical Entomology 44: 945-952. DOI: 10.1093/jmedent/44.6.945.
- Bradt D, Coburn L, Bradley KK, Noden BH. 2018. First record of Aedes japonicus japonicus in Oklahoma, 2017. Journal of the American Mosquito Control Association 34: 38-41. DOI: 10.2987/17-6690.1.
- Burkett-Cadena ND. 2013. Mosquitoes of the Southeastern United States. The University of Alabama Press: Tuscaloosa, AL. 208 pp.
- Butler M, Suom C, Lebrun RA, Ginsberg HS, Gettman AD. 2006. Effects of methoprene on oviposition by Aedes japonicus and Culex spp. Journal of the American Mosquito Control Association 22: 339-342. DOI: 10.2987/8756-971X(2006)22[339:EOMOOB]2.0.CO;2
- Byrd BD, Sither CB, Goggins JA, Kunze-Garcia S, Pesko KN, Bustamante DM, Sither JM, Vonesh JR, O'Meara GF. 2019. Aquatic thermal conditions predict the presence of native and invasive rock pool Aedes (Diptera: Culicidae) in the southern Appalachians, USA. Journal of Vector Ecology 44: 30-39. DOI: 10.1111/jvec.12326
- Connelly CR. 2004. Surveillance for mosquito-borne viruses. University of Florida, Institute of Food and Agricultural Science Extension EDIS Publication # ENY699.
- Egizi A, Kiser J, Abadam C, Fonseca DM. 2016. The hitchhiker's guide to becoming invasive: Exotic mosquitoes spread across a US state by human transport not autonomous flight. Molecular Ecology 25: 3033-3047. DOI: 10.1111/mec.13653.
- Georgia Mosquito Control Association (GMCA). 9 March 2018. Mosquito Maps, 2001-2017, Ochlerotatus japonicus. Accessed 22 April 2020.
- Haddow AD, Moulton JK, Gerhardt RR, McCuiston LJ, Jones CJ. 2009. Description of the egg of Ochlerotatus japonicus japonicus (Diptera: Culicidae) using variable pressure scanning electron microscopy. Journal of Medical Entomology 46: 9-14. DOI: 10.1603/033.046.0102.
- Harris MC, Dotseth EJ, Jackson BT, Zink SD, Marek PE, Kramer LD, Paulson SL, Hawley DM. 2015. La Crosse virus in Aedes japonicus japonicus mosquitoes in the Appalachian Region, United States. Emerging Infectious Diseases 21: 646-649. DOI: 10.3201/eid2104.140734.
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB. 1987. Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science 236: 1114-1116. DOI: 10.1126/science.3576225.
- Hutchinson M. Update on the invasive mosquito, Aedes j. japonicus in North America. 43rd Annual Conference of the Mid-Atlantic Mosquito Control Association and 52nd Annual Conference of the North Carolina Mosquito and Vector Control Association. 12-14 February 2018. Carolina Beach, NC.
- Ibañez-Justicia A, Teekema S, den Hartog W, Jacobs F, Dik M, Stroo A. 2018. The effectiveness of Asian bush mosquito (Aedes japonicus japonicus) control actions in colonized peri-urban areas in the Netherlands. Journal of Medical Entomology 55: 673-680. DOI: 10.1093/jme/tjy002.
- Jansen S, Heitmann A, Lühken R, Jöst H, Helms M, Vapalahti O, Schmidt-Chanasit J, Tannich E. 2018. Experimental transmission of Zika virus by Aedes japonicus japonicus from southwestern Germany. Emerging Microbes and Infections 7: 192. DOI: 10.1038/s41426-018-0195-x.
- Johnson KA, Brogren SJ, Crane DM, Lamere CA. 2010. Status of Aedes japonicus in the Metropolitan Mosquito Control District, Minnesota. Journal of the American Mosquito Control Association 26: 328-331. DOI: 10.2987/10-6018.1.
- Juliano SA, Lounibos PL. 2005. Ecology of invasive mosquitoes: Effects of resident species and on human health. Ecology Letters 8: 558-574. DOI: 10.1111/j.1461-0248.2005.00755.x
- Kampen H, Werner D. 2014. Out of the bush: The Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasites and Vectors 7: e59. DOI: 10.1186/1756-3305-7-59
- Kaufman MG, Fonseca DM. 2014. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annual Review of Entomology 59: 31-49. DOI: 10.1146/annurev-ento-011613-162012.
- Kaufman MG, Stanuszek WW, Brouhard EA, Knepper RG, Walker ED. 2012. Establishment of Aedes japonicus japonicus and its colonization of container habitats in Michigan. Journal of Medical Entomology 49:1307-17. DOI: 10.1603/me12061.
- Kaufman PE, Harrington LC, Waldron JK, Rutz DA. 2005. The importance of agricultural tire habitats for mosquitoes of public health importance in New York State. Journal of the American Mosquito Control Association 21: 171-176. DOI: 10.2987/8756-971X(2005)21[171:TIOATH]2.0.CO;2.
- Laird M, Calder L, Thornton RC, Syme R, Holder PW, Mogi M. 1994. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. Journal of the American Mosquito Control Association 10: 14-23.
- Lorenz AR, Walker ED, Kaufman MG. 2013. Does autocthonous primary production influence oviposition by Aedes japonicus japonicus (Diptera: Culicidae) in container habitats? Journal of Medical Entomology 50: 69-78. DOI: 10.1603/me12083.
- McKenzie BA, Stevens K, McKenzie AE, Bozic J, Mathias D, Zohdy S. 2019. Aedes vector surveillance in the Southeastern United States reveals growing threat of Aedes japonicus japonicus (Diptera: Culicidae) and Aedes albopictus. Journal of Medical Entomology 56: 1745-1749. DOI: 10.1093/jme/tjz115.
- Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, Hendrickx G, Zeller H, Van Bortel W, Schaffner F. 2015 An entomological review of invasive mosquitoes in Europe. Bulletin of Entomological Research 105: 637-663. DOI: 10.1017/S0007485315000103.
- Molaei G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. 2009. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. Journal of the American Mosquito Control Association 25: 210-214. DOI: 10.2987/09-0012.1.
- Peach DAH, Almond M, Pol JC. 2019. Modeled distributions of Aedes japonicus japonicus and Aedes togoi (Diptera: Culicidae) in the United States, Canada, and Northern Latin America. Journal of Vector Ecology 44: 119-129. DOI: 10.1111/jvec.12336.
- Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. 1999. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. Journal of the American Mosquito Control Association 15: 238-241.
- Reinert JF. 2000. New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. Journal of the American Mosquito Control Association 16: 175-188.
- Reinert JF, Harbach RE, Kitching IJ. 2006. Phylogeny and classification of Finlaya and allied taxa (Diptera: Culicidae: Aedini) based on morphological data from all life stages. Zoological Journal of the Linnean Society 148: 1-101. DOI: 10.1111/j.1096-3642.2006.00254.x.
- Riles MT, Smith JP, Burkett-Cadena N, Connelly CR, Morse GW Jr, Byrd BD. 2017. First record of Aedes japonicus in Florida. Journal of the American Mosquito Control Association 33: 340-344. DOI: 10.2987/17-6696.1.
- Rutledge CR, Day JF. 2002. Mosquito Repellents. University of Florida, Institute of Food and Agricultural Science Extension EDIS Publication # ENY-671.
- Sames W. 2020. Preparing for next generation of North American mosquito identification and bionomical publications. Wing Beats 31: 23-27.
- Sardelis MR, Dohm DJ, Pagac B, Andre RG, Turell MJ. 2002. Experimental transmission of eastern equine encephalitis virus by Ochlerotatus j. japonicus (Diptera: Culicidae). Journal of Medical Entomology 39: 480-84. DOI: 10.1603/0022-2585-39.3.480.
- Sardelis MR, Turell MJ, Andre RG. 2003. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j. japonicus. Journal of the American Mosquito Control Association 19: 159-162.
- Schaffner F, Chouin S, Guilloteau J. 2003. First report of Ochlerotatus japonicus japonicus in metropolitan France. Journal of the American Mosquito Control Association 191: 1-5.
- Schaffner F, Medlock JM, Van Bortel W. 2013. Public health significance of invasive mosquitoes in Europe. Clinical Microbiology and Infection. 19:685-92. DOI: 10.1111/1469-0691.12189..
- Schaffner F, Vazeille M, Kaufmann C, Failloux A, Mathis A. 2011. Vector competence of Aedes japonicus for chikungunya and dengue viruses. European Mosquito Bulletin 29: 141-142. DOI: 10.5167/uzh-53052.
- Schönenberger AC, Wagner S, Tuten HC, Schaffner F, Torgerson P, Furrer S, Mathis A, Silaghi C. 2016. Host preferences in host-seeking and blood-fed mosquitoes in Switzerland. Medical and Veterinary Entomology 30: 39-52. DOI: 10.1111/mve.12155.
- Scott J. 2003. The ecology of the exotic mosquito Ochlerotatus (Finlaya) japonicus japonicus (Theobald 1901) (Diptera: Culicidae) and an examination of its role in the West Nile virus cycle in New Jersey. PhD Dissertation, Rutgers University, New Jersey. 180 p.
- Smith JP, Taylor TJ, Cami I. Adams CI, Tennant RA,Cope EJ, Walsh J, Nomann B, Horn C, Johnson F, Hilson G, Golden C, O’Neal G, Kozak J, Armstrong K, Conyers L. 2020. Updated county mosquito species records for Northwest Florida. Journal of Florida Mosquito Control Association 67: 1-9.
- Takashima I, Rosen L. 1989. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus. Journal of Medical Entomology 26: 454-458. DOI: 10.1093/jmedent/26.5.454.
- Tanaka K, Mizusawa K, Saugstad ES. 1979. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara islands) and Korea (Diptera: Culicidae). Contributions of the American Entomological Institute 16: 1-987.
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. 2001. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. Journal of Medical Entomology 38: 130-134. DOI: 10.1603/0022-2585-38.2.130.
- Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, Andreadis TG, Blow JA. 2005. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. Journal of Medical Entomology 42: 57-62. DOI: 10.1093/jmedent/42.1.57.
- Turell MJ, Byrd BD, Harrison BA. 2013. Potential for populations of Aedes j. japonicus to transmit Rift Valley fever virus in the USA. Journal of the American Mosquito Control Association 29: 133-137. DOI: 10.2987/12-6316r.1.
- The Vermont Agency of Agriculture, Food & Markets (VAAFM). 2019. 2018 Mosquito Surveillance Report. Accessed 25 March 2020.
- Versteirt V, Schaffner F, Garros C, Dekoninck W, Coosemans M, Van Bortel W. 2009. Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. Journal of Medical Entomology 46: 1464-1467. DOI: 10.1603/033.046.0632.
- Winchester JC, Kapan DD. 2013. History of Aedes mosquitoes in Hawaii. Journal of the American Mosquito Control Association 29: 154-163. DOI: 10.2987/12-6292R.1.