common name: a mosquito
scientific name: Culex erraticus (Dyar and Knab, 1906) (Insecta: Diptera: Culicidae: Culicini)
Introduction - Synonymy - Distribution - Description - Life Cycle and Hosts - Medical and Veterinary Importance - Surveillance and Management - Selected References
Introduction (Back to Top)
Culex erraticus (Dyar and Knab, 1906) is a small dark mosquito that belongs to the subgenus Melanoconion. One of 26 subgenera of Culex, Melanoconion species are mostly found in the Americas, and most species occur in the tropics (Harbach 2009, Torres-Gutierez et al. 2018). Culex erraticus is found farther north than any other member of its subgenus and the only Melanoconion that exists in Canada.
Culex erraticus is typically found in permanent freshwater swamps but can be encountered in many other habitats and is widespread in Florida. Culex erraticus females can be aggressive biters (Ross 1947) and take blood meals from birds, mammals, amphibians, and reptiles (Robertson et al. 1993, Cohen et al. 2009, Bingham et al. 2014, Egizi et al. 2018). Because of its propensity for biting both birds and mammals, and its frequent infection with EEEV, Culex erraticus is a species of public health concern for its potential to transmit this arbovirus to humans (Cupp et al. 2003, Cohen et al. 2009, Bingham et al. 2016, Egizi et al. 2018).
Figure 1. Lateral view of an adult female Culex erraticus. Photograph by Nathan Burkett-Cadena, University of Florida.
Synonymy (Back to Top)
Culex degustator Dyar, 1921
Culex egberti Dyar and Knab, 1907
Culex homoepas Dyar and Ludlow, 1921
Culex peribleptus Dyar and Knab, 1918
Culex pose Dyar and Knab, 1918
Mochlostyrax erraticus Dyar and Knab, 1906
(ITIS 2021)
Distribution (Back to Top)
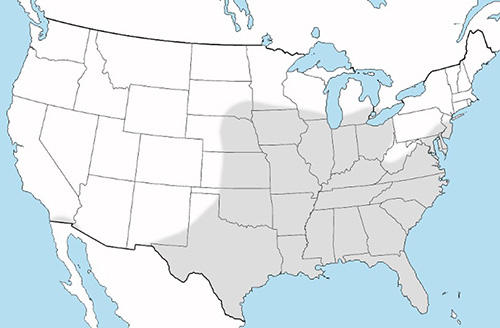
Culex erraticus is reported in northern South America, Central America, the eastern U.S. south of the Great Lakes, and southernmost California (Figure 2) (Burkett-Cadena 2013). This species is considered rare in the northeastern U.S., yet adult mosquitoes have been recorded as far north as Connecticut and Ontario, Canada (Petruff et al. 2020, Hunter et al. 2015). As annual temperatures rise, northward range expansion of Culex erraticus is expected (Hunter et al. 2015, Egizi et al. 2018). The northward geographical expansion of Culex erraticus may have serious consequences for increased transmission of EEEV.
Figure 2. Distribution of Culex erraticus in the United States. Map by Nathan Burkett-Cadena, University of Florida.
Culex erraticus ranges throughout Florida and likely occurs in all 67 Florida counties. In their updated distribution records for Florida mosquitoes, Riles and Connelly (2020) and Smith (2020) documented 18 (combined) new county records of Culex erraticus, indicating that this mosquito is widespread, but its range is poorly documented.
Description (Back to Top)
Eggs:
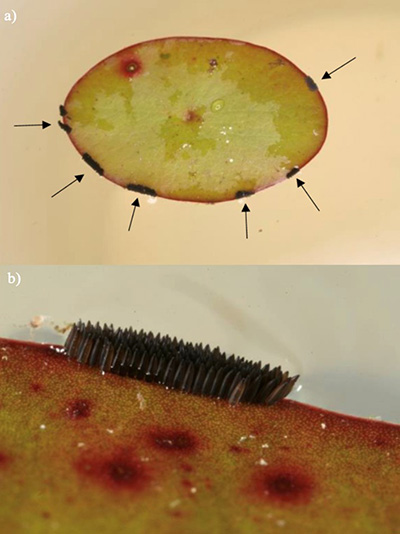
Figure 3. Egg rafts laid by Culex erraticus adult females on watershield (Brasenia) with a) six egg rafts on a leaf margin (denoted by arrows); b) close-up shot of a single raft viewed laterally. Photographs by Nathan Burkett-Cadena, University of Florida.
Females of many Culex species lay their eggs in floating clusters called “egg rafts” on the surface of the water. Unlike many other Culex species, Culex erraticus females lay their eggs in double or partial triple rows in a line or zigzag pattern (Mattingly 1976). Rather than laying them directly on water, Culex erraticus females usually oviposit their eggs along the edges of leaves of duckweed (Lemna), watershield (Brasenia) or other floating aquatic plants (Figure 3) (Burkett-Cadena 2013).
Larva:
Figure 4. Culex erraticus larvae showing variation in coloration of the head, thorax, and abdomen. Photograph by Nathan Burkett-Cadena, University of Florida.
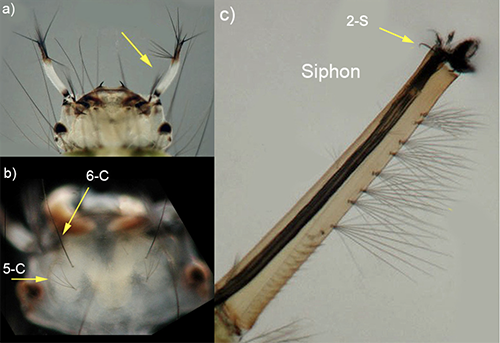
The larva of Culex erraticus is small. The color of the thorax and abdomen vary from light pale yellow, translucent green to black (Figure 4) (Burkett-Cadena 2013). On the larval head are two pale (usually white) antennae with dark tips (Figure 5a). Two pairs of setae (similar to hairs or bristles) are prominent on the middorsal surface of the head. Setae 6-C tend to be single (occasionally double) and are located anterior to setae 5-C, which are shorter and multibranched (Figure 5b) (Darsie and Ward 2016). The larva of Culex erraticus has a long and thin siphon that is slightly curved (Harrison et al. 2016), with small clusters of setae. The length and arrangement of setal tufts can be useful for distinguishing between subgenera and species of Culex. Larva of the subgenus Melanoconion have one or more pairs of subdorsal setae, and seta 2-S at the apex of the siphon is curved like a hook (Figure 5c) (Darsie and Ward 2016). On the ventral aspect (under-side) of the siphon, five pairs of setae, each with multiple branches, form a row extending roughly three-quarters of the length of the siphon (King et al. 1960). Culex erraticus can be distinguished from all other species of Melanoconion in Florida by the shape of comb scales, the length of the siphon, and the length of setae on the siphon (Darsie and Morris 2003).
Figure 5. Culex erraticus larva showing a) white color and dark tips of the antennae; b) dorsal surface of the larval head with single seta 6-C and shorter multibranched setae 5-C; c) the long thin siphon, subdorsal setae, strongly curved seta 2-S, and pairs of multiple branched setae on ventral aspect. Photographs by Michele Cutwa, University of Florida.
Pupa:
The pupa of Culex erraticus is small and dark, often with contrasting areas of dark and pale integument on the abdomen (Figure 6). The pupa continues development in the aquatic environment. The pupa does not feed during this short phase and is active if disrupted. Two short horn-like tubes, the respiratory trumpets, occur on either side of the cephalothorax (fused head and thorax) and are used for respiration in mosquito pupae. The brief pupal stage of development is not often used for species identification, although regional keys to species of pupae do exist (Darsie 2005).
Figure 6. Lateral view of pupa of Culex erraticus. Photograph by Kristin Sloyer, University of Florida.
Adult:
The Culex erraticus adult is a small and dark-colored mosquito. The palps are short, and the proboscis is long with a slightly swollen tip (Carpenter and LaCasse 1955). The palps and proboscis are covered in dark brown scales. The top of the head has broad pale scales on either side and along the edge of the compound eyes. These broad pale scales join at the point where the eyes meet, creating an inverted V-shape (Figure 7). Presence of broad pale scales on the dorsal aspect of the head, along with a lack of middorsal setae on the scutum (dorsum of the thorax) distinguishes the subgenus Melanoconion from other Culex subgenera in Florida (Darsie and Ward 2016). Culex erraticus also has a large patch of wide pale scales on the mesepimeron, a rectangular sclerite (plate) of the pleuron (lateral thorax) (Figure 8). The presence of this patch of pale scales differentiates Culex erraticus from other species of the subgenus Melanoconion in the U.S. (Darsie and Ward 2016). The scutum of Culex erraticus has narrow golden brown scales. While some mosquitoes, particularly species of Aedes and Psorophora, have unique patterns of dark and pale scales on the scutum that are useful for identification, in Culex erraticus the dark brown and golden scales do not form a distinctive pattern. The legs of Culex erraticus are dark with small light spots on the apex of the femurs. The wings are 2.5 to 3.0 mm in length and have narrow dark scales (Carpenter and LaCasse 1955). Lateral spots of pale scales on the abdomen are typically evident, while thin pale bands on tergites are typically narrow or absent (Ross 1947).
Figure 7. Dorsal view of Culex erraticus adult head showing the V-shaped patch of wide pale scales. Photograph by Nathan Burkett-Cadena, University of Florida.
Figure 8. Lateral view of head and thorax of Culex erraticus showing patch of broad scales on mesepimeron. Photograph by Nathan Burkett-Cadena, University of Florida.
Life Cycle and Hosts (Back to Top)
Culex erraticus is a holometabolous insect that goes through four stages of development: egg, larva, pupa, and adult. Adults are typically most abundant in summer and fall in the southeastern U.S. reaching peak numbers in July, August, and September. Mated (inseminated) adult females can overwinter in caves, hollow trees, and animal burrows (Breeland et al. 1961, Burkett-Cadena et al. 2011). When females emerge the following spring, they seek out a blood meal for nutrition for their developing eggs (King et al. 1960). Host-seeking Culex erraticus females are most active two hours prior to and after sunrise, and thirty minutes to one hour after sunset (Gray et al. 2011). Their activity is influenced by environmental factors such as temperature and humidity (Gray et al. 2011).
Host use of Culex erraticus varies both geographically and seasonally. In Alabama, Culex erraticus was found to bite primarily birds during the spring and then shift to biting mammals in the late summer (Oliveira et al. 2011, Burkett-Cadena et al. 2012). In southern Florida, where Culex erraticus is active year-round, Culex erraticus fed more heavily upon ectothermic hosts in the summer (20% of blood meals) than the winter and spring (<5%) (Bingham et al. 2014). A study in New Jersey documented that over ninety percent of Culex erraticus blood meals were of avian origin and less than seven percent were acquired from mammals (Egizi et al. 2018). A study in Tennessee, however, found that the majority of Culex erraticus blood meals were from mammals with white tailed deer accounting for more than seventy percent of the total blood meals (Cohen et al. 2009). Culex erraticus also bites reptiles (Figure 9) and amphibians (Cupp et al. 2003, Burkett-Cadena et al. 2008a).
Figure 9. Females of Culex erraticus biting a sleeping green anole lizard, Anolis carolinensis. Photograph by Nathan Burkett-Cadena, University of Florida.
Once the blood meal is digested, females use the nutrients to produce eggs which are laid on the edges of aquatic plants in freshwater hardwood swamps and marshes (Burkett-Cadena 2013). Eggs become fertilized upon oviposition and embryonic development occurs within a few days and its rate is temperature dependent. Upon hatching, larvae develop through four stages (instars) over a week to two-week period. Larval development is also influenced by temperature and was shown to be faster in water temperatures from 26 to 29°C (Breeland et al. 1961). The pupal phase is short and progresses to the adult stage in two to three days. A study in Tennessee found several overlapping generations of Culex erraticus each year, with oviposition and larval development ceasing at the onset of cooler temperatures in October (Breeland et al. 1961).
Medical and Veterinary Importance (Back to Top)
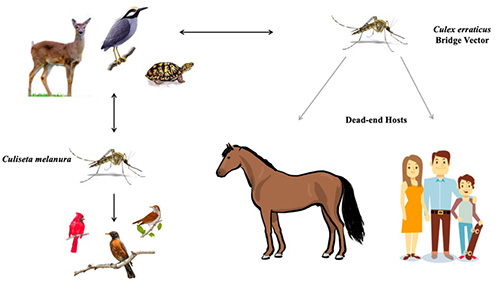
Culex erraticus is considered a bridge vector of EEEV in the U.S. (Figure 10) (Cupp et al. 2003, Cohen et al. 2009, Bingham et al. 2016, Egizi et al. 2018). This arbovirus can cause severe morbidity in human and equine hosts, and as many as one-third of the neuroinvasive cases can be fatal. Because EEEV primarily circulates in birds, Culex erraticus must take successive blood meals from both birds and mammals to transmit the virus to a human or horse (Gray et al. 2011, Vander Kelen et al. 2012, Burkett-Cadena 2012). The mosquito acquires EEEV when the mosquito takes a blood meal from a viremic bird. The virus then must infect specific tissues within the mosquito, specifically the midgut and salivary glands, before its bite becomes infectious. Once this occurs, the mosquito may transmit EEEV to a new host when it takes another bloodmeal (Burkett-Cadena 2012). If transmission occurs to humans or horses, the virus does not reach sufficient levels in the bloodstream for onward transmission to mosquitoes, so they are referred to as “dead-end hosts” (MacLachlan and Dubovi 2017).
Figure 10. Hypothetical transmission of eastern equine encephalitis virus with Culex erraticus as a bridge vector. Graphic by Mary Nordgulen, University of Florida.
Culiseta melanura is the primary vector of EEEV among birds but rarely feeds on humans (Scott and Weaver 1989, Komar and Spielman 1994). In contrast, Culex erraticus feeds frequently on mammals and tends to be more abundant than Culiseta melanura. For these reasons, Culex erraticus likely plays an important role in the transmission of EEEV to mammals (horses and humans) in areas where it is abundant (Bingham et al. 2016).
Although human disease due to infection with EEEV is rare in the U.S., the severity of neurological effects and high case fatality rate make it a noteworthy disease. Horses tend to develop symptoms first and may be early indicators of an outbreak. Head pressing is a clinical feature accompanied by fever, weight loss, rapid heartbeat, and lethargy. The case fatality rate for horses is as high as ninety percent (MacLachlan and Dubovi 2017). Although humans are less likely to become infected once infections occur, the elderly and young are at greatest risk for severe disease. Generally, symptoms are minor and may include fever, chills, malaise, and joint and muscle aches. Severe disease occurs when EEEV becomes neuroinvasive. In such cases symptoms vary and may include fever, headache, vomiting, seizures, drowsiness, and coma (CDC 2019). Thirty-eight human cases of neuroinvasive EEEV were reported to the Centers for Disease Control in 2019 (CDC 2020, Lindsey et al. 2020). Fifty percent of these cases were fatal and although these numbers are higher than previous years, they reflect the gravity of the disease. An annual EEEV vaccination is available for horses, but no vaccine is available for humans.
While not typically considered an important vector of West Nile Virus (WNV), Culex erraticus has been found naturally infected with this WNV in several states, including Florida (Hribar et al. 2004), Texas (Bolling et al. 2005), Alabama (Cupp et al. 2007), Tennessee (Cupp et al. 2007), and Louisiana (Unlu et al. 2010).
Culex erraticus is also considered an important host of saurian malaria in Florida. Based upon both laboratory and field evidence Klein et al. (1987, 1988) incriminated Culex erraticus in the transmission of Plasmodium floridense, a causative agent of “lizard malaria” that infects Anolis species lizards in Florida. Culex erraticus females entered traps baited with lizards, fed upon lizards in nature (Figure 9) and were able to support replication of the malaria parasite in the laboratory.
Surveillance and Management (Back to Top)
The potential of Culex erraticus to serve as a bridge vector for EEEV, as well as its abundance and recent geographical expansion are all reasons for surveillance and management of its populations. Resting shelter aspiration and CO2-baited CDC light traps are two effective methods for surveillance of Culex erraticus adults (Cupp et al. 2003, Burkett-Cadena et al. 2008b, Hunter et al. 2015). Bloodmeal analysis of collected specimens also provides crucial data for determining this species’ relative importance in transmitting EEEV, which may vary by location (Vander Kelen et al. 2012, Egizi et. al. 2018).
Currently, the most effective approaches to minimizing EEEV infection include avoidance of freshwater swamps where vector mosquitoes, including Culex erraticus, are active (D’Onofrio Jr. 2020). Biorational pesticides such as methoprene and Bacillus thuringiensis israelensis (Bti) are mosquito control methodologies for wide area management of larval habitats (Lawler 2017). Herbicides are sometimes applied to emergent and floating aquatic vegetation for the purpose of reducing habitat that harbors larvae of Culex erraticus and other mosquitoes that utilize this habitat (Mansonia titillans, Mansonia dyari, Anopheles crucians,and Anopheles quadrimaculatus). Use of insect repellents including DEET and Picaridin, wearing of light-colored garments that maximize coverage of extremities, and elimination of potential breeding sites are also recommendations to reduce exposure to mosquito bites and EEEV (CDC 2020). Horse owners should vaccinate their animals each year. Additional information on surveillance of mosquitoes and their control can be found at https://edis.ifas.ufl.edu/pdf%5CIN%5CIN104500.pdf.
Selected References (Back to Top)
- Bingham AM, Burkett-Cadena ND, Hassan HK, McClure CJ, Unnasch TR. 2014. Field investigations of winter transmission of eastern equine encephalitis virus in Florida. The American Journal of Tropical Medicine and Hygiene 1;91(4):685-93.
- Bingham AM, Burkett-Cadena ND, Hassan HK, Unnasch TR. 2016. Vector competence and capacity of Culex erraticus (Diptera: Culicidae) for eastern equine encephalitis virus in the southeastern United States. Journal of Medical Entomology 53(2):473-6.
- Bolling B, Kennedy JH, Zimmerman EG. 2005. Seasonal dynamics of four potential West Nile vector species in North-central Texas. Journal of Vector Ecology: journal of the Society for Vector Ecology 30(2):186-94.
- Breeland SG, Snow WE, Pickard E. 1961. Mosquitoes of the Tennessee Valley. Journal of the Tennessee Academy of Science 36:249-319.
- Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, Unnasch TR. 2008a. Bloodfeeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. The American Journal of Tropical Medicine and Hygiene 79(5):809-15.
- Burkett-Cadena ND. 2008b. Preference of female mosquitoes for natural and artificial resting sites. Journal of the American Mosquito Control Association 24(2):228-35.
- Burkett-Cadena ND, White GS, Eubanks MD, Unnasch TR. 2011. Winter biology of wetland mosquitoes at a focus of eastern equine encephalomyelitis virus transmission in Alabama, USA. Journal of Medical Entomology 48(5):967-73.
- Burkett-Cadena ND, Hassan HK, Eubanks MD, Cupp EW, Unnasch TR. 2012. Winter severity predicts the timing of host shifts in the mosquito Culex erraticus. Biology Letters 8(4):567-9.
- Burkett-Cadena ND. 2013. Mosquitoes of the Southeastern United States. University of Alabama Press, Tuscaloosa, Alabama. 208pp.
- Carpenter SJ, LaCasse WJ. 1955. Mosquitoes of North America (North of Mexico). University of California Press, Berkeley, California. 488pp.
- Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD). 2019. Eastern Equine Encephalitis (EEE). https://www.cdc.gov/easternequineencephalitis/tech/symptoms.html (25 May 2021)
- Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD). 2020. Eastern Equine Encephalitis (EEE). https://www.cdc.gov/easternequineencephalitis/tech/epi.html (29 January 2021)
- Cohen SB, Lewoczko K, Huddleston DB, Moody E, Mukherjee S, Dunn JR, Jones TF, Wilson R, Moncayo AC. 2009. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. The American Journal of Tropical Medicine and Hygiene 81(3):452-6.
- Cupp EW, Klingler K, Hassan HK, Viguers LM, Unnasch TR. 2003. Transmission of eastern equine encephalomyelitis virus in central Alabama. The American Journal of Tropical Medicine and Hygiene 68(4):495-500.
- Cupp EW, Hassan HK, Yue X, Oldland WK, Lilley BM, Unnasch TR. 2007. West Nile virus infection in mosquitoes in the mid-south USA, 2002-2005. Journal of Medical Entomology 44:117-25.
- Cutwa MM, O’Meara GF. 2012. Photographic Guide to Common Mosquitoes of Florida. Florida Medical Entomology Laboratory. (26 January 2021)
- Darsie RF, Morris CD. 2003. Keys to the adult females and fourth instar larvae of the mosquitoes of Florida (Diptera, Culicidae). Florida Mosquito Control Association.
- Darsie Jr. RF. 2005. Key to the pupae of the mosquitoes (Diptera: Culicidae) of Florida. Proceedings of the Entomological Society of Washington 107(4):892-902.
- Darsie Jr. RF, Ward RA. 2016. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. University Press of Florida, Gainesville, Florida. 398pp.
- D’Onofrio Jr. LE. 2020. Eastern equine encephalitis virus: A current and future danger. The Journal for Nurse Practitioners 16(10):744-6.
- Egizi A, Martinsen ES, Vuong H, Zimmerman KI, Faraji A, Fonseca DM. 2018. Using bloodmeal analysis to assess disease risk to wildlife at the new northern limit of a mosquito species. EcoHealth 15:543-54.
- Gray KM, Burkett-Cadena ND, Eubanks MD, Unnasch TR. 2011. Crepuscular flight activity of Culex erraticus (Diptera: Culicidae). Journal of Medical Entomology 48(2): 167-72.
- Harbach RE. 2009. Melanoconion Theobald, 1903. Mosquito Taxonomic Inventory. https://mosquito-taxonomic-inventory.info/simpletaxonomy/term/6182 (25 March 2021)
- Harrison BA, Byrd BD, Sither CB, Whitt PB. 2016. The Mosquitoes of the Mid-Atlantic Region: An Identification Guide. Publishing Xpress, Madison Heights, Michigan. 129p.
- Hribar L, Stark LM, Stoner RL, Demay DJ, Nordholt A, Hemmen MJ, Vlach J, Fussell EM. 2004. Isolation of West Nile Virus from mosquitoes (Diptera: Culicidae) in the Florida Keys, Monroe County, Florida. Caribbean Journal of Science 40(3):362-67.
- Hunter FF, Causarano J, Gasparotto A, Giordano BV. 2015. Establishment of Culex (Melanoconian) erraticus (Diptera: Culicidae) in southern Ontario, Canada. Journal of Medical Entomology 52(3):509-12.
- ITIS. 2021. Culex erraticus (Dyar and Knab, 1906) Taxonomic Serial No.:126466. The Integrated Taxonomic Information System. (26 January 2021)
- King WV, Bradley GH, Smith CN, McDuffie WC. 1960. A Handbook of the Mosquitoes of the Southeastern United States. U.S. Department of Agriculture, Agricultural Research Service Handbook, No. 173, Washington, DC. 194pp.
- Klein TA, Young DG, Telford Jr SR. 1987. Vector incrimination and experimental transmission of Plasmodium floridense by bites of infected Culex (Melanoconion) erraticus. Journal of the American Mosquito Control Association 1;3(2):165-75.
- Klein TA, Akin DC, Young DG, Telford Jr SR. 1988. Sporogony, development and ultrastructure of Plasmodium floridense in Culex erraticus. International Journal for Parasitology 1;18(6):711-9.
- Komar N, Spielman A. 1994. Emergence of eastern encephalitis in Massachusetts. Annals of the New York Academy of Sciences 740(1):157-68.
- Lawler SP. 2017. Environmental safety review of methoprene and bacterially-derived pesticides commonly used for sustained mosquito control. Ecotoxicology and Environmental Safety 139:335-43.
- Lindsey NP, Martin SW, Staples JE, Fischer M. 2020. Notes from the field: Multistate outbreak of eastern equine encephalitis virus - United States, 2019. Morbidity and Mortality Weekly Report 69(2):50-1.
- MacLachlan NJ, Dubovi EJ. 2017. Fenner’s Veterinary Virology: Togaviridae. Fifth Edition. Academic Press, Cambridge, Massachusetts. 511-524pp.
- Mattingly PF. 1976. Mosquito Eggs XXVIII. Mosquito Systematics 8(3):223-31.
- Oliveira A, Katholi CR, Burkett-Cadena N, Hassan HK, Kristensen S, Unnasch TR. 2011. Temporal analysis of feeding patterns of Culex erraticus in Central Alabama. Vector-Borne and Zoonotic Diseases 11(4):413-21.
- Petruff TA, McMillan JR, Shepard JJ, Andreadis TG, Armstrong PM. 2020. Increased mosquito abundance and species richness in Connecticut, United States 2001-2019. Scientific Reports 10(1):19287.
- Riles MT, Connelly CR. 2020. An update of the mosquito records of Florida counties, USA. Journal of the American Mosquito Control Association 36(2):107-11.
- Robertson LC, Prior S, Apperson CS, Irby WS. 1993. Bionomics of Anopheles quadrimaculatus and Culex erraticus (Diptera: Culicidae) in the Falls Lake Basin, North Carolina: Seasonal changes in abundance and gonotrophic status, and host-feeding patterns. Journal of Medical Entomology 30(4):689-98.
- Ross HH. 1947. The Mosquitoes of Illinois (Diptera, Culicidae). Illinois Natural History Survey Bulletin 24(1-4):1-96.
- Scott TW, Weaver SC. 1989. Eastern equine encephalomyelitis virus: Epidemiology and evolution of mosquito transmission. Advances in Virus Research 37:277-328.
- Smith JP, Taylor JT, Adams CI, Tennant Jr. RA, Cope E, Walsh J, Nomann B, Horn C, Johnson F, Hilson G, Golden C, O’Neal G, Kozak J, Armstrong K, Conyers L. 2020. Updated county mosquito species records for Northwest Florida. Journal of the Florida Mosquito Control Association 67(1):1-9.
- Torres-Gutierrez C, de Oliveira TMP, Emerson KJ, Sterlino Bergo E, Mureb Sallum MA. 2018. Molecular phylogeny of Culex subgenus Melanoconion (Diptera: Culicidae) based on nuclear and mitochondrial protein-coding genes. Royal Society Open Science 5: 171900.
- Unlu I, Kramer WL, Roy AF, Foil LD. 2010. Detection of West Nile virus RNA in mosquitoes and identification of mosquito blood meals collected at alligator farms in Louisiana. Journal of Medical Entomology 47:625-33.
- Vander Kelen PT, Downs JA, Stark LM, Loraamm RW, Anderson JH, Unnasch TR. 2012. Spatial epidemiology of eastern equine encephalitis in Florida. International Journal of Health Geographics 11(1):47.