common name: dagger nematode
scientific name: Xiphinema spp. (Cobb, 1913) Inglis, 1983 (Nematoda: Enoplea: Dorylaimia: Dorylaimina: Xiphinematinae)
Introduction - Distribution and Hosts - Life Cycle and Biology - Symptoms - Identification - Detection and Density Estimation - Economic Importance - Management - Selected References
Introduction (Back to Top)
Nematodes of the genus Xiphinema, commonly called dagger nematodes, parasitize plants. Many of these nematodes, the majority of them belonging to the Xiphinema americanum-group, can transfer viruses to plants during feeding (Taylor and Brown 1997, Gozel et al. 2006). Dagger nematodes can cause economic damage and death of host crops through feeding on the roots and also by spreading viral mosaic and wilting diseases (van Zyl et al. 2012, Jones et al. 2013). From a practical standpoint, it is a major challenge to control viral diseases in susceptible crops, partly because of a lack of resistant cultivars that should reduce populations of the virus vectors, Xiphinema spp. However, field studies have shown that some control measures, such as biofumigation and rotation of crops, targeting reduction in population of virus vectors, dagger nematodes, can be effective to some extent (Evans et al. 2007). Field surveys are required in order to implement appropriate and timely nematode management decisions that will minimize crop losses.
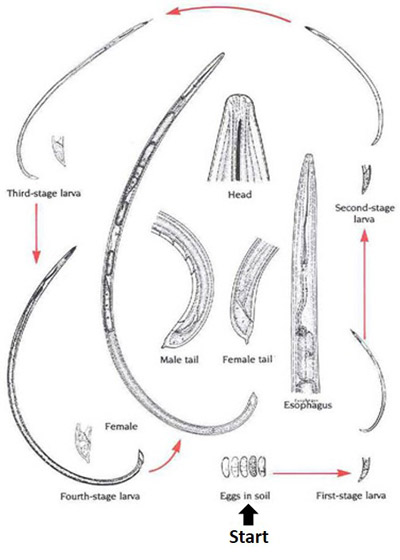
Figure 1. A typical life cycle of a dagger nematode, Xiphinema sp., with detailed features. Drawing by Nemaplex, University of California, Davis. Used with permission.
Distribution and Hosts (Back to Top)
Species of the genus Xiphinema are widely distributed in both temperate and tropical areas. They occur in South America, North America, Europe, Asia, Australia, New Zealand and Africa. In the U.S., Xiphinema spp. are classified as moderate pests on turfgrasses in landscapes in Massachusetts, Arkansas, California, the Carolinas (Robbins 1993, Ye et al. 2012) and Florida.
Five species belonging to the Xiphinema americanum-group have been detected on tomato, grape, oak, sea grape, pines, hackberry, Brazilian pepper and citrus in Florida and Morocco (Gozel et al. 2006, Mokrini et al. 2014). Other hosts include Sudangrass (sorghum), cotton, pearl millet, turfgrasses (Wick 2012, Ye et al. 2012), legumes, sugarcane, chili pepper, banana, sugar beet, corn (Shurtleff 1980), weeds, cassava (Rosa et al. 2014) and many more.
Life Cycle and Biology (Back to Top)
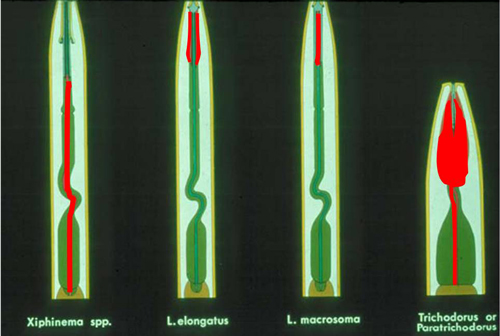
Dagger nematodes have six life cycle stages (Figure 1). Parthenogenesis, a form of reproduction that does not require males, is common in many, but not all species. Females lay eggs in soil. The life cycle of a dagger nematode is similar to other ectoparasitic, vermiform nematodes. Juveniles hatch from eggs and molt four times, increasing in size with each molt until they become adults. As vector-capable juveniles feed on virus-infected plants and mature into adults, they can acquire plant pathogenic viruses, commonly known as nepoviruses (nematode polyhedral viruses). The viruses form a lining in the pharynx-stylet tube (Figure 2) and are injected into root tissues during feeding (Lamberti and Roca 1987).
Dagger nematodes are ectoparasitic, which means that all stages, except eggs, attack and feed on the roots of the host plants. The nematode inserts its long stylet deep into the root while the body remains outside the root, in the soil. The stylet punctures cell walls as it penetrates plant tissues. During feeding, enzymes are secreted to digest plant cell contents. Plant parasitic nematodes produce enzymes such as cellulases, pectinases, hemi-cellulases, and chitinases, which are similar to those produced by bacteria and fungi (Jones et al. 2005, 2013), that digest and destroy root cells resulting in malformed root tissues (Figures 3 and 4). Root cells eventually collapse due to feeding.
Species of Xiphinema are sensitive to changes in soil temperature and moisture (Malek 1969) and will migrate vertically away from desiccating conditions in topsoil; most dagger nematodes can live and survive deep in soil (Feil et al. 1997).
Figure 2. Viral particles (in red) in the pharynx-stylet tubes of plant virus vector nematodes: A dagger nematode, Xiphinema; Needle nematodes, Longidorus elongatus and Longidorus macrosoma; Stubby nematodes, Trichodorus and Paratrichodorus. Drawing by Nemaplex, University of California, Davis. Used with permission.
Symptoms (Back to Top)
The damage dagger nematodes cause to root systems is similar to that of other plant ectoparasitic nematodes. The feeding at the meristematic root-tips destroys root cells (Figure 3) and reduces root volume. Terminal galling of roots of woody plants is common (Figure 4). The above-ground effects of damaged roots are stunted growth of crops and patchy fields.
Dagger nematodes transmit numerous viruses to plants. Cherry rasp leaf virus, Tomato ringspot virus, and Tobacco ringspot virus are some of the viruses transmitted by dagger nematodes during feeding. According to van Zyl et al. 2012, bermudagrass is a potential reservoir for GFLV (Grapevine fanleaf virus), which is transmitted by dagger nematodes. However, symptoms of viral infections, which include yellow mosaic and wilting shoots (Figures 5, 6, 7 and 8) are more visible in woody plants than in grasses because grasses show few or no symptoms (Hogmire 1995, Izadpanah et al. 2003, Palomares-Rius et al. 2012).
Figure 3. A dagger nematode, Xiphinema sp., feeding at fig root tip. Photograph by Nemaplex, University of California, Davis, USA. Photograph used with permission.
Figure 4. Terminal galling of grapevine root caused by a dagger nematode, Xiphinema sp. Photograph by Pablo Castillo, Institute for Sustainable Agriculture, CSIC, Córdoba, Spain.
Figure 5. Yellow mosaic disease caused by Grapevine fanleaf virus transmitted by dagger nematode, Xiphinema sp. Photograph by Pablo Castillo, Institute for Sustainable Agriculture, CSIC, Córdoba, Spain.
Figure 6. Leaves of Viking red currants showing symptoms of Tomato ringspot virus transmitted by dagger nematodes, Xiphinema sp. Photograph by Joseph Postman, Plant Pathologist, USDA-ARS National Clonal Germplasm Repository, Corvallis, Oregon.
Figure 7. Damaged leaves of Pelargonium hortorum infected with Tomato ringspot virus transmitted by dagger nematodes, Xiphinema sp. Photograph by the State Plant Pathology Institute of Denmark Archive, Bugwood.org. Photograph used with permission.
Figure 8. Potato plants heavily infected with Tobacco ringspot virus transmitted by dagger nematodes, Xiphinema sp. Photograph by the International Potato Center Archive, Bugwood.org. Photograph used with permission.
Identification (Back to Top)
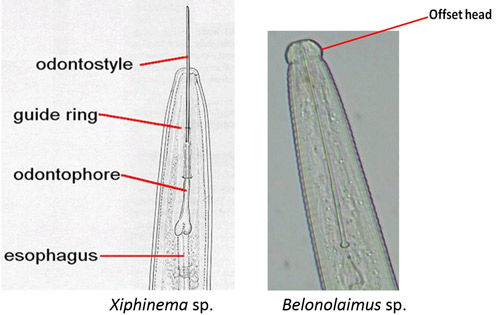
Xiphinema spp. belong to the sub-family Xiphinematinae, which has diverse groups of species (Coomans et al. 2001). An adult dagger nematode has a long body (2 to 6 mm) and a flat and smooth lip region (Goodey et al. 1960, Brown and Topham 1984, 1985, Siddiqi and Lenne 1990) (Figure 9). The head is not offset (Figure 10).
Species of the genus Xiphinema have a long stylet called an odontostyle (Decraemer and Gerart 2006). The stylet has no stylet knobs but rather flanges, which support (anchor) the basal part of the odontophore (the rear part of the long stylet) (Figures 10 and 11D). The guiding ring in the middle holds the long stylet in position.
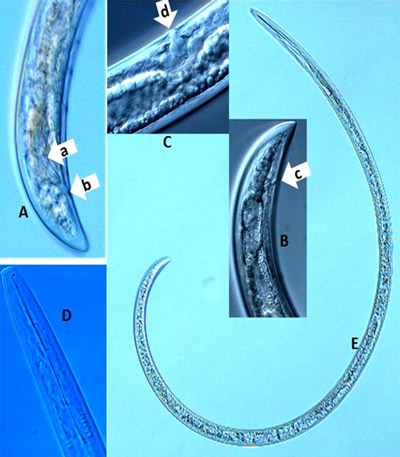
It is difficult to use the tail region (Figure 11A, B) to identify dagger nematodes, but the sexes can be differentiated using their tail regions. An adult male has paired spicules (Figure 11A, arrow a) and cloaca (Figure 11A, arrow b). Adult female dagger nematodes have an anus at the tail region (Figure 11B, arrow c) and a vulva (Figure 11C, arrow d) located mid-body, but at different locations depending on species. It is not possible to distinguish sexes of juveniles because sex organs are not developed. The tail ends of both adult males and females of some species, for example Xiphinema vuittenezi, Xiphinema israeliae, etc., have a small mucron (Luc et al. 1964, 1982).
Figure 9. Schematic diagram showing detailed morphological features of a dagger nematode, Xiphinema sp. Drawing by Nemapix (vol. 2) from Patton et al. 2010. Used with permission.
Figure 10. A drawing comparing anterior regions of a dagger nematode, Xiphinema sp. to a sting nematode, Belonolaimus sp. Sting nematode has an offset head region and a stylet with basal knobs; dagger nematodes do not have an offset head region and the basal region of the stylet has flanges. Photographs from Nemaplex, University of California, Davis. Used with permission.
Figure 11. Photographs of a dagger nematode, Xiphinema sp. A: lateral view of a male tail region with paired spicules (arrow a) and cloaca (arrow b); B: lateral view of anus (arrow c) at the tail region of a female; C: lateral view of vulva (arrow d) of a female; D: a head region showing full stylet; E: a full body length view. Photographs by Tesfamarian Mengistu, Entomology and Nematology Department, University of Florida.
Detection and Density Estimation (Back to Top)
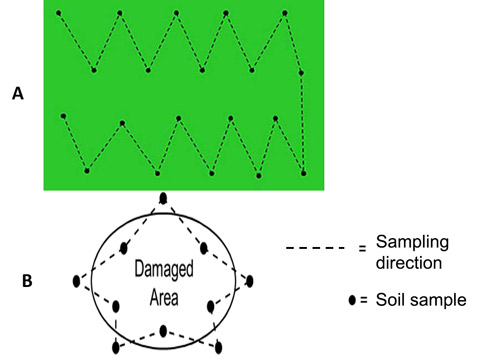
In infested fields, nematode problems occur in patches. Figure 12 shows a typical example of how plant parasitic nematodes should be sampled in a field. As many samples as possible are taken along the marked lines in a zig-zag manner. In dry conditions, samples should be taken from depths up to 60 cm using wider shovels. Soil samples taken from shallow depths may not include dagger nematodes. However in wet seasons, soil samples can be taken up to 40 cm depth using core samplers because dagger nematodes move upward when moisture content of topsoil is high.
Dagger nematodes are retrieved from soil samples using the Baermann funnel methods (OEPP/EPPO 2009, 2013), and sugar floatation method (Lawrence and Zehr 1978) among others. Population densities of dagger nematodes extracted from samples are analyzed or diagnosed for management decision-making.
Figure 12. Patterns of sampling in fields A) for survey B) with suspected plant parasitic nematode problems in symptomatic spot. Drawings (A) by William T. Crow, Landscape Nematology Specialist, UF/IFAS Extension, Entomology and Nematology Department, University of Florida and (B) by Ministry of Agriculture, Food and Rural Affairs, Ontario, Canada, used with permission.
Economic Importance (Back to Top)
Population densities in landscape areas can range from 0 to nearly 500 per 100cm3 of soil but the direct attack and feeding on roots by dagger nematodes cause only moderate damage to susceptible landscape plants (e.g., creeping bentgrass) (Moseley et al. 2010, Patton et al. 2010, Ye et al. 2012). However, Xiphinema spp. are the eighth most economically important plant parasitic nematode group to agricultural crops worldwide (Jones et al. 2013). Damage to roots causes up to 65% root weight loss and this can result in severe yield reduction (Anonymous 2014). Viruses transmitted to food crops such as tomato, grapevine, pepper, cassava, and potato can cause more than 50% crop losses because the virus limits plant development (Evans et al. 2007, Anonymous 2014). As a result, virus vectoring dagger nematodes are on quarantine lists for many countries (Nicol et al. 2011). There are no cultivars available resistant to dagger nematodes.
Management (Back to Top)
The main objectives of managing plant parasitic nematodes are to keep their population densities below damage thresholds and minimize economic losses. Several recommendations have been suggested to manage plant parasitic nematodes. Unfortunately, no single method is completely effective. Combining different management practices is more effective for reducing plant parasitic nematode density than using any method alone.
Dagger nematodes spread to fields through the use of contaminated equipment, planting of infested plants/sod and contaminated irrigation water or run-off. So, sanitation is critical. Chabrier and Quénéherve (2008) observed that man-made ditches intercepting run-off across steep slopes effectively reduced incidence of plant parasitic nematodes in fields. Clean equipment such as tractors, planters, and ridgers should be used in order to minimize transfer of nematodes to fields.
Fumigation has proved to be a good strategy. White, black or red plastic sheets are normally used to cover the soil surface following soil fumigation. However, clear plastic sheets allow sunlight to pass through and generate heat that kills and reduces plant parasitic nematodes in soils before planting (McSorley and Gill 2010).
Decomposing organic mulch or straw, green manure and trap crops such as Crotalaria and Sesbania can be used to encourage fungi like Pochonia that kill plant parasitic nematodes (Wildmer et al. 2002). Mustard bran and mustard seed meal, as well as products from Euphorbia spp., can be incorporated into soils because these plants contain nematicidal elements (Moseley et al. 2010).
Marigolds (Tagetes spp.) can be grown as cover crops and are effective in reducing populations of nematodes. According to Evans et al. (2007), marigolds used in crop rotation plans significantly reduced populations of dagger nematodes and severity of soybean severe stunt virus in soybeans. Marigolds produce root exudates (containing alpha-terthienyl) that suppress plant parasitic nematodes, pathogenic fungi, and bacteria (Wang et al. 2007, Krueger et al. 2010).
Robust and healthy plants are more tolerant of plant parasitic nematodes than highly stressed plants. Application of fertilizers and adequate water are important to maintain healthy plants.
Weed control is important because some weeds are also good hosts to dagger nematodes and the plant virus they transmit (Nemabase 2013).
For current management of plant parasitic nematodes on crops and landscape plants, refer to the latest recommendations on EDIS.
Selected References (Back to Top)
- Anonymous. 2014. Xiphinema index and grapevine fanleaf virus disease.
- Brown DJF, Topham PB. 1984. A comparison of reported variation in the morphometrics of Xiphinema diversicaudatum (Nematoda: Dorylaimida) and the effects of some methods of preparing specimens for examination by optical microscopy. Nematologia Mediterranea 12: 169-186.
- Brown DJF, Topham PB. 1985. Morphometric variability between populations of Xiphinema diversicaudatum (Nematoda: Dorylaimoidea). Revue de Nematologie 8: 15-26
- Chabrier C, Quénéherve P. 2008. Preventing nematodes from spreading: A case study with Radopholus similis (Cobb) Thorne in a banana field. Crop Protection 27: 1237-1243.
- Coomans A, Huys R, Heyns J, Luc M. 2001. Character analysis, phylogeny and biogeography of the genus Xiphinema Cobb, 1973 (Nematoda: Longidoridae). Annales du Musée Royal de l’ Afrique (Zoologie), Tervuren, Belgique 287: 1-239.
- Decraemer W, Gerart E. 2006. Ectoparasitic nematodes. In Plant Nematology. Perry RN, Moens M. (eds). CABI, Wallingford, UK, pp 153-184.
- Evans TA, Miller LC, Vasilas BL, Taylor RW, Mulrooney RP. 2007. Management of Xiphinema americanum and soybean severe stunt in soybean using crop rotation. Plant Disease 91: 216-219.
- Feil H, Westerdahl BB, Smith RJ, Verdegaal P. 1997. Effects of seasonal and site factors on Xiphinema index populations in two California vineyards. Journal of Nematology 29: 491-500.
- Goodey JB, Peacock FC, Pitcher RS. 1960. A redescription of Xiphinema diversicaudatum (Micoletzky, 1923, 1927) Thorne, 1939 and observations on its larval stages. Nematologica 5: 127-135.
- Gozel U, Lamberti F, Duncan L, Agostinelli A, Rosso L, Nguyen K, Adams BJ. 2006. Molecular and morphological consilience in the characterization and delimitation of five nematode species from Florida belonging to the Xiphinema americanum-group. Nematology 8: 521-532.
- Hogmire Jr HW. 1995. Mid-Atlantic orchard monitoring guide, NRAES-75. Plant and Science Life Publishing, Ithaca, New York.
- Izadpanah K, Zaki-Aghl M, Zhang YP, Daubert SD, Rowhani A. 2003. Bermuda grass as a potential reservoir host for grapevine fanleaf virus. Plant Disease 87: 1179-1182.
- Jones JT, Furlanetto C, Kikuchi T. 2005. Horizontal gene transfer from bacteria and fungi as a driving force in the evolution of plant parasitism in nematodes. Nematology 7: 641-646.
- Jones JT, Haegeman A, Etienne GJD, Hari SG, Helder J, Michael GKJ, Kikuchi T, Rosa ML, Juan EPR, Wesemael, WML, Perry RN. 2013. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology 4: 946-961.
- Krueger R, Dover KE, McSorley R, Wang KH. 2010. Marigolds (Tagetes spp.) for nematode management. Electronic Data Information Source, University of Florida, Institute of Food and Agricultural Sciences, Gainesville, FL. ENY 056.
- Lamberti F, Roca F. 1987. Present status of nematodes as vectors of plant viruses, pp 321-328. In Vistas on Nematology. Veech JA, Dickson DW. (eds). Society of Nematologists, Hyattsville, Maryland.
- Lawrence EG, Zehr, EI. 1978. Improvement of techniques for determining populations of Macroposthonia xenoplax in dry soil. Phytopathology 68: 1102-1105.
- Luc M, Brown DJF, Cohn E. 1982. Xiphinema israeliae n. sp. (Nematoda: Dorylaimoidea). Revue Nématologie 5: 233-239.
- Luc M, Lima MB, Weischer B, Flegg JJM. 1964. Xiphinema vuittenezi n. sp. (Nematoda: Dorylaimidae). Nematology 10(1): 151-163. DOI 10.1163/187529264X00781.
- Malek RB. 1969. Population fluctuations and observations of the life cycle of Xiphinema americanum associated with cottonwood (Populus deltoides) in South Dakota. 2011 Proceedings of the Helminthological Society 36: 270-274.
- McSorley R, Gill HK. 2010. Introduction to soil solarization. ENY 062, Entomology and Nematology Department, Florida Cooperative Extension Service, IFAS, University of Florida, Gainesville, FL.
- Mokrini F, Abbad AF, Waeyenberge L. 2014. First report of the dagger nematode Xiphinema diversicaudatum in citrus orchards in Morocco. Plant Disease 98: 575-575.
- Moseley D, Patton A, Bateman R, Kirkpatrick T. 2010. Controlling nematodes on golf courses. Cooperative Extension Service, University of Arkansas, MP481, 12 pp.
- Nemabase (http://plpnemweb.ucdavis.edu/nemaplex/Taxadata/G143S1.HTM#Hosts). 2013. Host range of a genus Xiphinema and species of plant-feeding nematodes.
- Nicol JM, Turner SJ, Coyne DL, den Nijs L, Hockl S, Maafi ZT. 2011. Current nematode threats to the world agriculture. In Genomics and molecular genetics of plant-nematode interactions. Jones J, Gheysen G, Fenoll C. (eds). DOI 10.1007/978-94-007-0434-3_2. Springer, Ankara, Turkey.
- OEPP/EPPO. 2009. EPPO standards PM7/95(1) diagnostics: Xiphinema americanum sensu lato. OEPP/EPPO Bulletin 39: 382-392.
- OEPP/EPPO. 2013. Diagnostics: Nematode extraction. OEPP/EPPO Bulletin 43: 471-495.
- Palomares-Rius JE, Gutiérrez-Gutiérrez C, Cantalapiedra-Navarrete C, Castillo P. 2012. Prevalence and diversity of grapevine fanleaf virus in southern Spain. Plant Pathology 61: 1032-1042.
- Patton A, Moseley D, Bateman R, Kirkpatrick T. 2010. Nematode management in lawns. Cooperative Extension Service, University of Arkansas, Agriculture and Natural Resources, FSA6141, 8 pp.
- Robbins RT. 1993. Distribution of Xiphinema americanum and related species in North America. Journal of Nematology 25: 344-348.
- Rosa O, Magrinelli J, de Oliveira SA, Luis JA, Amauri S, de Oliveira G, Marcelo C. 2014. Plant parasitic nematodes on cassava cultivated in the Brazilian Amazon. Acta Amazonica 44: 271-277.
- Shurtleff MC. 1980. Compendium of corn diseases. The American Phytopathological Society, St. Paul, Minnesota, 105 pp.
- Siddiqi MR, Lenne JM. 1990. Xiphinema llanosum and Trophurus vultus, two new plant nematode species from pasture soils in Colombia. Journal of Nematology 22: 262-267.
- Taylor CE, Brown DJF. 1997. Nematode vectors of plant viruses. CABI Publishing, Wallingford, UK.
van Zyl S, Vivier MA, Walker MA. 2012. Xiphinema index and its relationship to grapevines: A review. South African Journal of Enology and Viticulture 33: 21-32.
- Wang KH, Cerruti RH, Antoon P. 2007. Protecting crops from nematode pests: Using marigold as an alternative to chemical nematicides. Plant Disease, UH-CTAHR, PD-35: 1-6.
- Wick R. 2012. Nematodes on golf greens. Agriculture and Landscape Program. The Center for Agriculture, Food and the Environment, University of Massachusetts Amherst.
- Wildmer TL, Mitkowski NA, Abawi GS. 2002. Soil organic matter and management of plant parasitic nematodes. Journal of Nematology 34: 289-295.
- Ye W, Yongsan Z, Lane T, Martin S, Matt M, Hanafy F. 2012. Nematode distribution in turfgrass in the Carolinas 2011 survey. Carolina green, pp 23-40.