common name: a sand fly
scientific name: Lutzomyia longipalpis (Lutz and Neiva) (Insecta: Diptera: Psychodidae: Phlebotominae)
Introduction - Synonymy - Distribution - Description - Life Cycle - Hosts - Medical Importance - Management - Selected References
Introduction (Back to Top)
The true sand flies are small dipterans in the family Psychodidae, sub-family Phlebotominae. These flies are densely covered with setae, have long slender legs, and broad and pointed wings that are held erect at rest (Lane 1993, Rutledge and Gupta 2009). The term sand fly also is applied to biting midges that belong to the family Ceratopogonidae and to black flies that are members of the family Simuliidae (Rutledge and Gupta 2009). Several phlebotomine species are vectors of the protozoan parasites in the genus Leishmania, that are the causal agents of leishmaniasis. There are three main forms of leishmaniasis: cutaneous, muco-cutaneous and visceral (WHO 2015).
Figure 1. Adult (A) female and (B) male Lutzomyia longipalpis (Lutz and Neiva), a sand fly. Photographs by Cristina Ferro, Instituto Nacional de Salud, Colombia.
Visceral leishmaniais is the most severe form of the disease, and is fatal to the human or dog host if untreated (Chappuis et al. 2007). Visceral leishmaniasis is caused by Leishmania donovani in East Africa and India and Leishmania infantum (syn. Leishmania chagasi) in southern Europe, northern Africa, Latin America, the Middle East, Central Asia and China (Palatnik-de-Sousa and Day 2011, Vilhena et al. 2014). In Latin America this parasite is transmitted by Lutzomyia longipalpis, Lutzomyia evansi (Nuñez-Tovar) (Travi et al. 1990) and Lutzomyia cruzi (Mangabeira) (Santos et al. 1998). Lutzomyia longipalpis sensu latu is a species complex (reviewed by Uribe 1999) consisting of at least three difficult to differentiate species that each utilize this name. The complex has a wide distribution, ranging from Mexico to Argentina, and it is considered the main vector of visceral leishmaniasis in much of Central and South America (Young and Duncan 1994).
Synonymy (Back to Top)
Phlebotomus longipalpis Lutz and Neiva 1912
Phlebotomus otamae Nunez-Tovar 1924
Phlebotomus almazani Galliard 1934
Flebotomus longipalpis Barretto 1947
Lutzomyia longipalpis Theodor 1965
Distribution (Back to Top)
Lutzomyia longipalpis has a wide distribution that extends from southern Mexico to northern Argentina (Young and Duncan 1994). Within this zone, the flies primarily occur in tropical dry forests (González et al. 2006) that are between 0 and 1000 meters above sea level, have an average temperature of 24° C, and an annual rainfall between 1000 and 2000 mm (Holdridge 1967). Tropical dry forests occur in climates that are warm year-round, and receive several rain events per year, with dry seasons that may last several months (WWF 2015).
Description (Back to Top)
Adults: Phlebotomine sand fly adults are silvery-brown or yellow, covered by setae, and have a humped thorax, slender legs, and long, almost cylindrical antennal segments (Figure 1 A, B). They have pointed and broad wings that are held erect at rest (like a vertical V) (Lane 1993, Rutledge and Gupta 2009). Both sexes feed on sugars found in plant sources, such as nectar (Chaniotis 1974). Females have biting mouthparts and suck blood (Lane 1993). Males are readily recognized because they have prominent external structures, called coxites, which extend past the tip of the abdomen (Rutledge and Gupta 2009).
Lutzomyia longipalpis females have a total body length of approximately 2 mm and their wings are around 2.3 mm long and 0.65 mm wide. Males have shorter and narrower wings (Lutz and Neiva 1912).
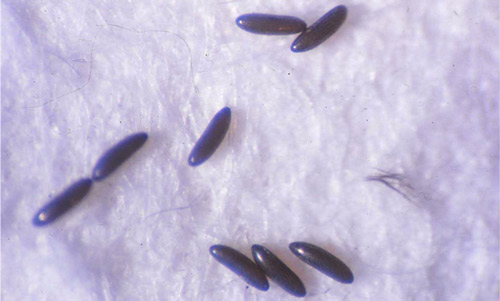
Eggs: Sand fly eggs are dark and elliptical (Young and Duncan 1994). They are about 400 µm long, with fine markings in their surface. These patterns may vary among species and include forms with ridges, polygons, and pits (Rutledge and Gupta 2009). The surface of Lutzomyia longipalpis eggs has parallel and unconnected ridges (Ward and Ready 1975, De Almeida et al. 2004) (Figure 2).
Figure 2. Eggs of Lutzomyia longipalpis (Lutz and Neiva), a sand fly. Photograph by Cristina Ferro, Instituto Nacional de Salud, Colombia.
Larvae: Sand flies have four larval instars. First instars have a single pair of caudal setae or long hair-like structures that stick out from their posterior end. These setae measure around 0.51 mm long and 0.08 mm wide (Leite and Williams 1997). The subsequent instars have two pairs of caudal setae. Third instars are differentiated from second instars by an increase in body and head capsule size. Fourth instar larvae are approximately 3.29 mm long and 0.42 mm wide and the posterior part of the 9th abdominal segment is pigmented (Leite and Williams 1996) (Figure 3). Larvae feed on organic material in the soil (Ferro et al. 1997).
Figure 3. Fourth instar larvae of Lutzomyia longipalpis (Lutz and Neiva), a sand fly. Note the pigmentation of the posterior part of the 9th segment and the presence of two pairs of caudal setae. Photograph by Cristina Ferro, Instituto Nacional de Salud, Colombia.
Pupae: Newly formed sand fly pupae are white, turning yellow and finally almost black before adult emergence (Figure 4). The compressed larval exoskeleton with its setae can be seen attached to the pupa. In their natural environment, pupae are attached to substrates such as dead leaves or stones (Young and Duncan 1994). Lutzomyia longipalpis pupae are approximately 2.5 mm long (Leite et al. 1991).
Figure 4. Newly formed Lutzomyia longipalpis (Lutz and Neiva) pupae. Photograph by Cristina Ferro, Instituto Nacional de Salud, Colombia.
Life Cycle (Back to Top)
The developmental time of each Lutzomyia longipalpis generation is typically six to seven weeks, but a generation may be completed more quickly. Females require a blood meal to mature eggs. The time from blood meal to oviposition is five to nine days. Once eggs are laid, they require four to nine days to hatch. Larvae develop in nine to 24 days and the pupal stage lasts for approximately 10 days. Under laboratory conditions, most females die after oviposition in the first gonotrophic cycle (Killick-Kendrick et al. 1977, Luitgards-Moura et al. 2000) (Figure 5).
In an established colony of Lutzomyia longipalpis from which 24 consecutive generations had been reared, the average number of eggs laid per female in the later generations was 50 (Killick-Kendrick et al. 1977). Luitgards-Moura et al. (2000) reported on aspects related to productivity for four generations of a Lutzomyia longipalpis laboratory colony. The mean number of eggs laid per female varied from a low of 23.6 (third generation-F3) to a maximum of 39.9 (first generation-F1).
Differences in the percentage of females that take a blood meal and in the number of eggs laid per female have been attributed to the blood meal source. Macedo-Silva et al. (2014) reported that blood from an anesthetized guinea pig or horse was best to support oviposition. Human blood, given through an artificial membrane, also supported sand fly oviposition. Sand flies would not feed on cat and those feeding on opossum did not produce eggs.
The ecology of sand flies in the immature stage is difficult to study, in part because of the extreme difficulty of finding immature forms in nature. Some developmental sites that have been reported for Lutzomyia longipalpis include animal corrals (Deane and Deane 1957) such as pigpens (Ferro et al. 1997) and chicken sheds (Casanova et al. 2013), as well as at the bases of trees (Ferro et al. 1997, Casanova et al. 2013), and in rock piles (Deane and Deane 1957, Ferro et al. 1997).
Figure 5. A female Lutzomyia longipalpis (Lutz and Neiva) and her eggs. Photograph by Cristina Ferro, Instituto Nacional de Salud, Colombia.
Hosts (Back to Top)
Lutzomyia longipalpis is an opportunistic blood feeder. Several studies have demonstrated that Lutzomyia longipalpis feeds on humans (Morrison et al. 1993, de Oliveira et al. 2008, Macedo-Silva et al. 2014). Humans are an important source of blood in some regions (de Oliveira et al. 2008), while in others, female flies are not particularly anthropophilic and feed predominantly on large domestic mammals such as cattle and swine (Morrison et al. 1993). Other blood meal sources include birds, dogs (Morrison et al. 1997, de Oliveira et al. 2008, Macedo-Silva et al. 2014), opossums, horses, reptiles (Morrison et al. 1997) and armadillos (Macedo-Silva et al. 2014).
Medical Importance (Back to Top)
There are three main forms of leishmaniasis: cutaneous, muco-cutaneous and visceral (WHO 2015). Lutzomyia longipalpis is considered the main vector in most areas in which visceral leishmaniasis transmission occurs in Latin America (Young and Duncan 1994). Visceral leishmaniasis, in which vital organs are affected, is the most severe form of the disease, and is fatal to the host if untreated. Its symptoms are fever, fatigue, weakness, loss of appetite, weight loss, and the enlargement of lymph nodes, spleen and liver (Chappuis et al. 2007).
There are two types of visceral leishmaniasis: zoonotic and anthroponotic. In zoonotic visceral leishmaniasis, the causal agent/parasite is Leishmania infantum (syn. Leishmania chagasi) and it is transmitted from an animal reservoir, typically a dog, to a sand fly and finally to a human. In anthroponotic visceral leishmaniasis, the causal agent is Leishmania donovani and it is transmitted from human to sand fly to human, without the involvement of another mammal. Zoonotic visceral leishmaniasis is transmitted in southern Europe, North Africa, Latin America, The Middle East, Central Asia and China. Anthroponotic visceral leishmaniasis occurs in East Africa and India (Chappuis et al. 2007, Palatnik-de-Sousa and Day 2011, Vilhena et al. 2014).
In Central America, Lutzomyia longipalpis also has been associated with an atypical form of cutaneous leishmaniasis, caused by Leishmania infantum (syn. Leishmania chagasi). In an endemic area of visceral leishmaniasis in Honduras, Lutzomyia longipalpis has been implicated as a possible vector of this unusual cutaneous leishmaniasis, in which patients develop small papules, mainly on the face, that do not ulcerate (Ponce et al. 1991). In Costa Rica, similar cases also have been reported. Although, Lutzomyia longipalpis is the most common sand fly species in the area, Leishmania parasites have not been isolated from the sand flies (Zeledón et al. 1989).
Every year an estimated 300,000 cases of visceral leishmaniasis are reported worldwide. This disease is fatal if left untreated and approximately 20,000 deaths from the disease occur each year. The recommended treatment regimen varies according to the region and the Leishmania species present (WHO 2015). Early diagnosis and treatment of cases is essential (Palatnik-de-Sousa and Day 2011). Available treatments options have challenges relating to efficacy, adverse effects, costs and access to medicines (Moore and Lockwood 2010).
Management (Back to Top)
The control of sand flies is focused on adults because developmental habitats are difficult to find, making immature control impractical. To be effective, insecticide applications targeting sand flies must be long-lasting (residual) insecticides that are applied in or on dwellings and animal shelters to kill adult flies before they bite humans and other animals. Insecticide use should be combined with an adequate knowledge of the vector and a suitable public health infrastructure (Maroli and Khoury 2006). The first attempt to control sand flies in South America was reported in Peru in 1944 and focused on bartonellosis, a disease caused by a different pathogen transmitted by these sand flies. The use of insecticide for Lutzomyia longipalpis control was first attempted in Brazil in the 1950s (Alexander and Maroli 2003).
Current control measures for sand flies include the use of insecticide-treated bed nets (Courtenay et al. 2007). This is the most sustainable measure to reduce leishmaniasis when transmission occurs inside dwellings and the resting sites of sand flies are unknown. The use of recommended chemical repellents and protective clothing for sand fly control seems to be the only preventative measure available outside the home (Alexander and Maroli 2003).
Dogs are the only domestic reservoir of Leishmania infantum. Insecticide-impregnated dog collars have been used and have shown a potent antifeeding and insecticidal effect against these flies (Reithinger et al. 2001). However, the effectiveness of this control measure is diminished when collars are lost or not replaced when insecticides are depleted (Alexander and Maroli 2003). Alternatively, spot-on repellents have shown efficacy (Miró et al. 2007, Ferroglio et al. 2008). The presence of stray dogs in many areas and the occurrence of transmission throughout the year hamper management of this disease and require permanent monitoring by public health authorities (Alexander and Maroli 2003).
Selected References (Back to Top)
- Alexander B, Maroli M. 2003. Control of phlebotomine sandflies. Medical and Veterinary Entomology 17: 1-18.
- Casanova C, Andrighetti MTM, Sampaio SMP, Marcoris MLG, Colla-Jacques FE, Prado AP. 2013. Larval breeding sites of Lutzomyia longipalpis (Diptera: Psychodidae) in visceral leishmaniasis endemic urban areas in southeastern Brazil. PLoS Neglected Tropical Diseases 7: e2443.
- Chaniotis BN. 1974. Sugar-feeding behavior of Lutzomyia trapidoi (Diptera: Psychodidae) under experimental conditions. Journal of Medical Entomology 11: 73-79.
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling R, Alvar J, Boelaert M. 2007. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nature Reviews Microbiology 5: 873-882.
-
Courtenay O, Gillingwater K, Gomes PAF, Garcez LM, Davies CR. 2007. Deltamethrin-impregnated bednets reduce human landing rates of sandfly vector Lutzomyia longipalpis in Amazon households. Medical and Veterinary Entomology 21: 168-176. - De Almeida DN, Oliveira RS, Brazil BG, Soares MJ. 2004. Patterns of exochorion ornaments on eggs of seven South American species of Lutzomyia sand flies (Diptera: Psychodidae). Journal of Medical Entomology 41: 819-825.
- de Oliveira AG, Marassá AM, Consales CA, Dorval ME, Fernandes CE, de Oliveira GR, Brazil RP, Galati EA. 2008. Observations on the feeding habits of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) in Campo Grande, an endemic area of visceral leishmaniasis in Mato Grosso do Sul, Brazil. Acta Tropica 107: 238-241.
- Deane, LM, Deane MP. 1957. Observacoes sobre abrigos e criadouros de flebotomos no noroeste do estado do Ceara. Revista Brasileira de Malariologia e Doenças Tropicais 9: 225-246.
- Ferro C, Pardo R, Torres M, Morrison A. 1997. Larval microhabitats of Lutzomyia longipalpis (Diptera: Psychodidae) in an endemic focus of visceral leishmaniasis in Colombia. Journal of Medical Entomology 34: 719-728.
- Ferroglio E, Poggi M, Trisciuoglio A. 2008. Evaluation of 65% permethrin spot-on and deltamethrin-impregnated collars for canine Leishmania infantum infection prevention. Zoonoses and Public Health 55: 145-148.
- González C, Cabrera OL, Munstermann LE, Ferro C. 2006. Distribución de los vectores de Leishmania infantum (Kinetoplastida: Tryposomatidae) en Colombia. Biomédica 26: 64-72.
- Holdridge LR. 1967. Life zone ecology. San José de Costa Rica: Tropical Science Center, 206 pp.
- Killick-Kendrick R, Leaney AJ, Ready PD. 1977. The establishment, maintenance and productivity of a laboratory colony of Lutzomyia longipalpis (Diptera: Psychodidae). Journal of Medical Entomology 13: 429-440.
- Lane RP. 1993. Sandflies (Phlebotominae). In Medical Insects and Arachnids, Lane RP, Crosskey RW (Eds.) Chapman & Hall, London, pp. 78-119.
- Leite ACR, Williams P, Santos MC. 1991. The pupa of Lutzomyia longipalpis (Diptera: Psychodidae-Phlebotominae). Parassitologia 33: 477-484.
- Leite ACR, Williams P. 1996. Description of the fourth instar larva of Lutzomyia longipalpis, under scanning electron microscopy. Memórias do Instituto Oswaldo Cruz 91: 571-578.
- Leite ACR, Williams P. 1997. The first instar larva of Lutzomyia longipalpis (Diptera: Phlebotomidae). Memórias do Instituto Oswaldo Cruz 92: 197-203.
- Luitgards-Moura JF, Castellón Bermúdez EG, Rosa-Freitas MG. 2000. Aspects related to productivity for four generations of a Lutzomyia longipalpis laboratory colony. Memórias do Instituto Oswaldo Cruz 95: 251-257.
- Lutz A, Neiva Z. 1912. Contribuição para o conhecimento das espécies do gênero Phlebotomus existentes no Brasil. Memórias do Instituto Oswaldo Cruz 4: 84-95.
- Macedo-Silva VP, Martins DR, De Queiroz PV, Pinheiro MP, Freire CC, Queiroz JW, Dupnik KM, Pearson RD, Wilson ME, Jeronimo SMB, Ximenes MF. 2014. Feeding preferences of Lutzomyia longipalpis (Diptera: Psychodidae), the sand fly vector, for Leishmania infantum (Kinetoplastida: Trypanosomatidae). Journal of Medical Entomology 51: 237-244.
- Maroli M, Khoury C. 2006. Current approaches to the prevention and control of leishmaniasis vectors. Veterinary Research Communications 30: 49-52.
- Miró G, Gálvez R, Mateo M, Montoya A, Descalzo MA, Molina R. 2007. Evaluation of the efficacy of a topically administered combination of imidacloprid and permethrin against Phlebotomus perniciosus in dog. Veterinary Parasitology 143: 375-379.
- Moore EM, Lockwood DN. 2010. Treatment of visceral leishmaniasis. Journal of Global Infectious Diseases 2: 151-158.
- Morrison A, Ferro C, Tesh R. 1993. Host preferences of the sand fly Lutzomyia longipalpis at an endemic focus of American visceral leishmaniasis in Colombia. American Journal of Tropical Medicine and Hygiene 49: 68-75.
- Palatnik-de-Sousa CB, Day MJ. 2011. One Health: the global challenge of epidemic and endemic leishmaniasis. Parasites & Vectors 4: 197. DOI: 10.1186/1756-3305-4-197
- Ponce C, Ponce E, Morrison A, Cruz A, Kreutzer R, McMahon-Pratt D, Neva F. 1991. Leishmania donovani chagasi: New clinical variant of cutaneous leishmaniasis in Honduras. Lancet 337: 67-70.
- Reithinger R, Teodoro U, Davies CR. 2001. Topical insecticide treatments to protect dogs from sand fly vectors of leishmaniasis. Emerging Infectious Diseases 7: 872-876.
- Rutledge LC, Gupta RK. 2009. Moth flies and sand flies. In Medical and Veterinary Entomology, Mullen R, Durden LA (Eds). 2nd Edition, Academic Press, London, pp, 153-168.
- Santos SO, Arias JR, Ribeiro AA, Hoffmann MP, Freitas RA, Malacco MAF. 1998. Incrimination of Lutzomyia cruzi as a vector of American visceral leishmaniasis. Medical and Veterinary Entomology 12: 315-317.
- Travi BL, Velez ID, Brutus L, Segura I, Jaramillo C, Montoya J. 1990. Lutzomyia evansi, an alternate vector of Leishmania chagasi in a Colombian focus of visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 84: 676-677.
- Uribe S. 1999. The status of the Lutzomyia longipalpis species complex and possible implications for Leishmania transmission. Memórias do Instituto Oswaldo Cruz 94: 729-734.
- Vilhena H, Granada S, Oliveira AC, Schallig HD, Nachum-Biala Y, Cardoso L, Baneth G. 2014. Serological and molecular survey of Leishmania infection in dogs from Luanda, Angola. Parasites & Vectors 7: 114. DOI: 10.1186/1756-3305-7-114
- Ward RD, Ready PA. 1975. Chorionic sculpturing in some sandfly eggs (Diptera: Psychodidae). Journal of Entomology Series A 50: 127-134.
- World Health Organization (WHO). (2015). Leishmaniasis. http://www.who.int/leishmaniasis/en/ (27 Feb 2015).
- World Wildlife Fund (WWF). (2015). Tropical and Subtropical Dry Broadleaf Forest Ecoregions http://wwf.panda.org/about_our_earth/ecoregions/about/habitat_types/selecting_terrestrial_ecoregions/habitat02.cfm (15 Feb 2015).
- Young DG, Duncan MA. 1994. Guide to identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Memoirs of the American Entomological Institute. Num. 54, 881 pp.
- Zeledón R, Hidalgo H, Víquez A, Urbina A. 1989. Atypical cutaneous leishmaniasis in a semiarid region of north-west Costa Rica. Transactions of the Royal Society of Tropical Medicine and Hygiene 83: 786.