common name: Asian honey bee (suggested common name)
scientific name: Apis cerana Fabricius (Insecta: Hymenoptera: Apidae)
Introduction - Distribution - Description - Life Cycle - Biology - Economic Impact - Management - Acknowledgements - Selected References
Introduction (Back to Top)
The Asian honey bee, Apis cerana Fabricius (Fig. 1), occurs in southern Asia. There are eight recognized subspecies of Apis cerana, including Apis cerana cerana and Apis cerana indica. Both Apis cerana cerana and Apis cerana indica are managed for honey production and crop pollination, similarly to how western honey bees, Apis mellifera L., are used in the United States. Apis cerana is a natural host to two major honey bee pests, Varroa destructor and Nosema ceranae, both of which also infect Apis mellifera. Furthermore, Apis cerana has expanded beyond it native range and there is particular concern about its spread into Australia.
Figure 1. An adult worker (non-reproductive female) of Apis cerana. Photograph by Charles Lam via Flickr, CC-BY-SA-2.0.
All honey bee species (members of the genus Apis) share many morphological, behavioral and physiological characteristics. Here we summarize those characteristics and highlight some of the defining differences between Apis cerana and Apis mellifera.
Distribution (Back to Top)
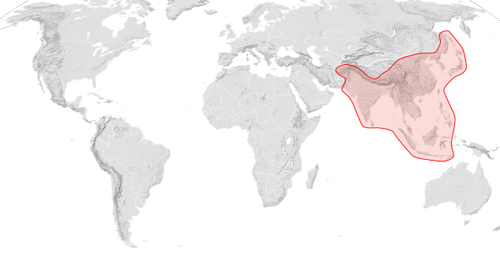
Apis cerana occurs across south and southeastern Asia up to Russia in the north. Its natural distribution extends to Japan and stretches as far west as Afghanistan (Fig. 2). Similar to Apis mellifera, there are many subspecies of Apis cerana. These subspecies tolerate a wide range of temperatures from cold, temperate, to tropical ecosystems.
Apis cerana was introduced intentionally into Papua New Guinea in the late 1970s. Subsequently, Apis cerana has continued to expand its range into the Solomon Islands and Australia. The Australian government has begun educational programs to teach the identification of Apis cerana, and destruction of identified wild nests is common practice in efforts to cease its spread within the country. However, the success of these programs has been limited and Apis cerana seems to be well established in tropical and subtropical Queensland (northeastern Australia).
Figure 2. The native distribution of Apis cerana. The introduced distribution of Apis cerana (Papua New Guinea, The Solomon Islands, and northeastern Australia) is not illustrated in this map. Figure by Sémhur Canuckguy, via Wikimedia Commons.
Description (Back to Top)
The physical characteristics of Apis cerana are very similar to that of Apis mellifera. Apis cerana adults have branched (plumose) hairs on their bodies to assist in pollen collection. Their workers (non-reproductive females, Fig. 2) have a corbiculum (pollen basket) on their hind legs to transport pollen. The worker's ovipositor (an organ for egg laying) has been modified into a stinger and adults are yellow and black in color.
The abdominal stripes (tomenta) of Apis cerana are more pronounced than those of Apis mellifera, and Apis cerana workers have four abdominal stripes, whereas Apis mellifera workers have three abdominal stripes. Worker body size varies among subpopulations throughout Apis cerana's geographic distribution. Generally, workers of the southern subspecies of Apis cerana are smaller than Apis mellifera workers. However, workers of northern subspecies of Apis cerana are larger than some of the African subspecies of Apis mellifera.
Queens are the reproductive females of the colony and are larger than the workers, with abdomens enlarged to accommodate their developed reproductive organs (Fig. 3). Most queens are somewhat darker than the workers.
Figure 3. An adult Apis cerana queen (reproductive female) surrounded by workers. Photograph by Azman, CC BY-SA-3.0.
Males of Apis cerana are called drones. Each colony produces significantly fewer drones than they do workers. Drones have large eyes that meet at the top of their head, no stinger, and their abdomen is thick and blunt at the end. This gives the drones more of a blunt-ended body rather than the pointed, aerodynamic body shape that is seen in the female castes (Fig. 4).
Figure 4. An adult Apis cerana drone (male) on a section of uncapped honey comb. The drone is centered in the image and several adult workers surround the drone. Photograph by Niko Koeniger, Martin–Luther-Universität, Institut für Biologie, Bereich Zoologie.
Most colonies consist of one queen, thousands of workers, and numerous drones. The queen's only responsibility within the colony is to lay eggs and she is the mother of all the workers present in the colony. The workers preform all hive maintenance tasks including: tending to the brood (eggs, larvae, and pupae), cleaning, foraging, and producing honey. These tasks are divided among the workers by age, a phenomenon called temporal or age-related polyethism. Drones are produced to mate with a queen from another colony, and therefore are only produced during the reproductive season.
Life Cycle (Back to Top)
Apis cerana is holometabolous; meaning individuals undergo four distinct life stages (egg, larva, pupa, and adult).
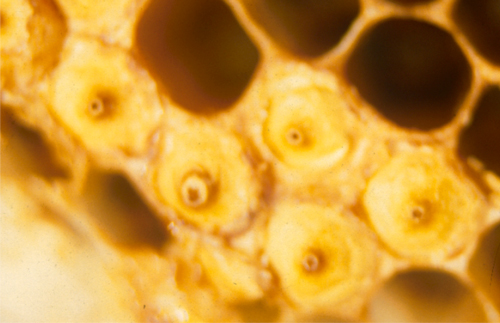
Eggs: The queen lays a single egg in each brood cell. Eggs are small, white, and oval in shape (Fig. 5). Larvae emerge from eggs after three days.
Figure 5. An egg and first instar honey bee larvae. The egg is located in the top center cell. First instar larvae can be seen in the surrounding cells. Photograph by Alexander Wild.
Larvae: Newly hatched larvae curl into the shape of a C at the bottom of the cell. Larvae are white in color, blind, and legless, with a wet shine (Fig. 5 and 6). The larvae are fed brood food and/or royal jelly within their cells until they are large enough to pupate, at which time the adult worker bees cap the larval cells.
Figure 6. Final instar larvae in their cells. Photograph by Alexander Wild.
Pupae: The larvae molt into pupae beneath the wax cappings and the pupal cells are undisturbed until pupation is complete. The wax capping of the drone pupal cells in Apis cerana have a distinctive pore (Fig. 7). The purpose of this pore is not yet known. However, another species of honey bee, Apis koschevnikovi Buttel-Reepen, constructs a similar pore on the drone pupal capping.
Figure 7. Capped drone pupal cells, and several empty cells from which adults have emerged already. Photograph by Nikolaus Koeniger, Martin–Luther-Universität, Institut für Biologie, Bereich Zoologie.
Adults: Following their pupal development, the new adults chew their way out of their capped cells.
Honey bees are considered superorganisms, wherein the whole colony is considered the biological unit rather than are the individual bees. Colony-level reproduction is referred to as swarming and it generally happens in the spring and summer. However, swarming may be more frequent in tropical areas where climate is more favorable year-round. Abundant resources (nectar and pollen) and large colony size are thought to be the primary triggers for swarming. To initiate swarming, the colony will raise 10 to 20 daughter queens. When the daughter queens are in the late pupal stage, the mother queen and up to two-thirds of the adult workers leave the colony in search of a place to establish a new colony (typically a cavity like a hollow tree).
Once the daughter queens emerge as adults, they fight until only one queen remains. The remaining queen is unmated and must leave the colony on mating flights where she will mate with upwards of 20 drones. The sperm the queen collects is stored in a special organ called the spermatheca and is used to fertilize eggs for the rest of her reproductive lifespan.
The sex of each bee is determined via haplo-diploid sex determination. In this system, the queen decides the sex of her offspring by laying unfertilized or fertilized eggs. Unfertilized eggs (no paternal genetic contribution) develop into drones, and fertilized eggs (both maternal and paternal genetic contributions) develop into females. The female larvae further differentiate into workers or queens based on the diet they are fed. Female larvae that are fed the standard diet of pollen and nectar (brood food) become adult workers. In contrast, female larvae that are fed royal jelly develop into queens.
Biology (Back to Top)
Apis cerana prefers to nest in enclosed cavities, like a hollow tree. Apis cerana colonies are typically smaller than Apis mellifera colonies and tend to prefer smaller nest cavities. Apis cerana colonies consist of approximately 34,000 bees, while Apis mellifera colonies average between 20,000 and 60,000 bees. Both Apis cerana and Apis mellifera build multiple combs arranged parallel to one another. However, Apis cerana does not use propolis, a glue-like material, to seal cracks and holes in their hives, as does Apis mellifera.
Behaviorally, Apis cerana is almost indistinguishable from Apis mellifera. Apis cerana is known to be a very docile, gentle, and even somewhat timid, but there can be large differences in their defensiveness depending on season and region. Apis cerana colonies tend to swarm and abscond (abandon a hive location) more frequently than do Apis mellifera colonies. When fanning to circulate air at the colony entrance, Apis cerana workers face the opposite direction that Apis mellifera workers face when performing the same task. Apis mellifera workers face the hive entrance, but Apis cerana workers face away from the colony entrance (Fig. 8). Finally, Apis cerana has several unique responses to disturbances including: fast and sudden lateral body shaking of workers, the production of a hissing sound, and heat balling.
Figure 8. Apis cerana workers fanning at a colony entrance. Note that the fanning workers are facing away from the colony entrance. Figure by Peter Jaeger, used with permission from Nikolaus Koeniger, Martin–Luther-Universität, Institut für Biologie, Bereich Zoologie.
Heat balling is a unique defense that Apis cerana has evolved to kill predatory hornets, particularly Vespa simillima xanthoptera Smith. Once a hornet is discovered, several hundred bees surround the hornet in a tight ball and vibrate their thoracic muscles to produce heat. The Apis cerana workers are able to raise the temperature inside the ball to an average of 46°C for approximately 20 minutes (Fig. 9). This temperature is high enough to kill the hornet inside, but not high enough to kill the bees, who can tolerate temperatures up to 48 and 50°C. Apis mellifera workers also will surround a hornet, but they are not able to raise the temperature as high as can Apis cerana. Instead Apis mellifera workers primarily sting the hornet and are much less effective at eliminating the intruder.
Figure 9. Apis cerana workers forming a defensive heat ball around two hornets. Photograph by Takahashi via GFDL, CC BY-SA-2.1 JP.
Economic Impact (Back to Top)
Apis cerana is an important bee to beekeepers in Asia, especially in poor communities. There are initiatives to teach beekeeping as a long-term employment opportunity in these communities. Apis cerana is kept by beekeepers in diverse mountainous areas that can be difficult to reach. Yet Apis cerana can thrive in these areas as they are adapted for the environment. Apis cerana also is managed in other areas within its native range.
Many beekeepers are transitioning to Apis mellifera management because the average Apis cerana colony produces less honey than does the average Apis mellifera colony. However, In many parts of Asia, Apis mellifera can survive only under intense care and protection offered by the beekeeper, while the vast majority of Apis cerana colonies still live wild and naturally in balance with a vast array of predators, pest, and parasites (e.g., hornets, sun bears, Varroa). One example of this is that Apis mellifera must be treated with pesticides for Varroa control, whereas Apis cerana is a natural host of Varroa and does not require beekeeper intervention. Therefore, Apis cerana colonies can be used to produce organic honey.
Honey is only one of the many marketable products produced by Apis cerana colonies. Some beekeepers specialize in the production of wax, pollen, and/or provide pollination services. Apis cerana is known to be an excellent pollinator of many crops including: spice crops, fruits, nuts, oilseeds, cauliflower, okra, and onion. In some situations, they are considered to be a superior pollinator compared to Apis mellifera.
Several factors make Apis cerana efficient pollinators, the first being their smaller foraging range. A smaller range means that each worker spends more time with the same plants and has higher floral fidelity than does Apis mellifera. The smaller colony size of Apis cerana is also advantageous, as it makes them easy to transport and manage. Furthermore, Apis cerana has a longer daily foraging period than does Apis mellifera. Apis cerana starts foraging earlier in the morning and continues to forage later into the evening than does Apis mellifera. Also, Apis cerana will forage at lower temperatures than will Apis mellifera. Unfortunately, there is no current estimate on the economic contribution of Apis cerana as a pollinator.
Management (Back to Top)
The management of Apis cerana as a business venture is not a new practice; colonies have been kept in simple log hives and other basic nest structures for over 2,000 years. There are several regional standardized hive boxes used for Apis cerana. The size of the hive and the bee space (spacing of the wax comb frames) is adapted to the size of the Apis cerana subspecies in that region as well as to the size of the colony being housed. Additionally, Apis cerana beekeepers are beginning to adopt the Langstroth hive (the most popular honey bee hive in the United States), along with top-bar style hives.
Within its native range, Apis cerana is in need of conservation efforts. Deforestation, loss of nest sites, pathogens, and increased pesticide use have contributed to a steady decline of the Apis cerana population. Furthermore, the replacement of Apis cerana management by Apis mellifera management in many areas affects the native flora in addition to the bee population. While the Apis mellifera colonies may be more profitable for honey production, they are not equivalent pollinators of native plants. Efforts to increase the management of Apis cerana colonies have had some success.
In contrast to its need for conservation within its native range, Apis cerana may be considered an invasive species in other parts of the world. For example, the progression of Apis cerana south past Papua New Guinea into Australia is being monitored extensively. The Australian government is concerned that Apis cerana could become an invasive species, with negative effects on the introduced Apis mellifera population. Australian bees are not infected with many of the pests that Apis cerana is known to host. Therefore, Australian beekeepers fear that the establishment of an Apis cerana population could lead to the introduction of Varroa, and Nosema ceranae.
Acknowledgements (Back to Top)
Reviews by Nikolaus Koeniger and Gudrun Koeniger, Martin–Luther-Universität, Institut für Biologie, Bereich; Howard Frank, Entomology and Nematology Department, University of Florida.
Selected References (Back to Top)
- Abrol DP. 2013. Asiatic honeybee Apis cerana: Biodiversity conservation and agricultural production. Springer Science+Business Media. 1016 pp.
- Ellis JD, Zettel Nalen CM. 2013. Varroa destructor Anderson and Trueman (Arachnida: Acari: Varroidae). University of Florida, IFAS, Entomology and Nematology Department, Featured Creatures, EENY-473. (8 December 2014).
- Engel MS. 1999. The taxonomy of recent and fossil honey bees (Hymenoptera: Apidae: Apis). Journal of Hymenoptera Research 8: 165-196.
- Hadisoesilo S, Otis GW, Meixner M. 1995. Two distinct populations of cavity-nesting honey bees in South Sulawesi, Indonesia. Journal of the Kansas Entomological Society 68: 399-407.
- Koetz AH. 2013. Ecology, behavior, and control of Apis cerana with a focus on relevance to the Australian incursion. Insects 4: 558-592. doi: http://dx.doi.org/10.3390/insects4040558
- Koetz AH. 2013. The Asian honey bee (Apis cerana) and its strains - with special focus on Apis cerana Java genotype. Department of Agriculture, Fisheries, and Forestry, State of Queensland. (8 December 2014).
- Moritz RFA, Southwick EE. 1992. Bees As Superorganisms: An Evolutionary Reality. Springer-Verlag, New York, USA. 395 pp.
- Mortensen AN, Burleson S, Chelliah G, Johnson K, Schmehl DR, Ellis JD. 2014. Tropilaelaps spp. Delfinado & Baker (Arachnida: Mesostigmata: Laelapidae). University of Florida, IFAS, Entomology and Nematology Department, Featured Creatures, EENY 604. (8 December 2014).
- Mortensen AN, Schmehl DR, Ellis JD. 2013. Apis mellifera Linnaeus, and subspecies (Insecta: Hymenoptera: Apidae). University of Florida, IFAS, Entomology and Nematology Department, Featured Creatures, EENY 568. (8 December 2014).
- Oldroyd BP, Nanork P. 2008. Conservation of Asian honey bees. Apidologie 40: 296-312. dio: http://dx.doi.org/10.1051/apido/2009021
- Oldroyd BP, Wongsiri S. 2006. Asian honey bees: Biology, conservation, and human interactions. Harvard University Press, Cambridge, Massachusetts, USA. 360 pp.
- Ono M, Okada I, Sasaki M. 1987. Heat production by balling in the Japanese honeybee, Apis cerana japonica as a defensive behavior against the hornet, Vespa simillima xanthoptera (Hymenoptera: Vespidae). Experientia 43: 1031-1034. doi: http://dx.doi.org/10.1007/BF01952231
- Rath W. 1999. Co-adaptation of Apis cerana Fabr. and Varroa jacobsoni Oud. Apidologie 30: 97-110. doi: http://dx.doi.org/10.1051/apido:19990202
- Ruttner F, Woyke J, Koeniger N. 1972. Reproduction in Apis cerana 1. Mating Behavior. Journal of Apicultural Research 11: 140-146.
- Ruttner F, Woyke J, Koeniger N. 1973. Reproduction in Apis cerana 2. Reproductive organs and natural insemination. Journal of Apicultural Research 12: 21-34.
- Srinivasan MR. 2004. Biodiversity of honeybees. Advances in management of productive insects, CAS Training program. Tamil Nadu Agricultural University, Coimbatore. 14 pp.
- Triplehorn CA, Johnson NF, Borror DJ. 2005. Borror and DeLong’s introduction to the study of insects. Thompson Brooks/Cole, Belmont, California, USA. 864 pp.
- Winston ML. 1987. The biology of the honey bee. Harvard University Press, Cambridge, Massachusetts, USA. 281 pp.
- Yang M. 2009. Studies of mixed-species colonies of honey bees, Apis cerana and Apis mellifera. Ph.D. dissertation, Rhodes University. 138 pp.