common name: papaya fruit fly (suggested common name)
scientific name: Toxotrypana curvicauda Gerstaecker (Insecta: Diptera: Tephritidae)
Introduction - Synonymy - Distribution - Description - Life Cycle - Damage - Management - Selected References
Introduction (Back to Top)
The papaya fruit fly, Toxotrypana curvicauda Gerstaecker, is the principal insect pest of papaya (Carica papaya L.) throughout the tropical and subtropical areas of the New World. The insect was introduced into Florida in 1905, most likely from the West Indies on papaya shipments. It first became established in the Florida Keys and Miami, then spread throughout the state wherever papayas are grown. Papaya fruit fly larvae and adults have been found in Florida in every month of the year. Although originally considered to be monophagous, infesting only wild and cultivated papaya, the insect has also been reported on mango and milkweed in Florida, and other plant species in Mexico.
Figure 1. Adult female papaya fruit fly, Toxotrypana curvicauda Gerstaecker. Photograph by Doug Caldwell, University of Florida.
Synonymy (Back to Top)
Mikimyia furcifera Bigot.
Distribution (Back to Top)
The papaya fruit fly is distributed throughout the Caribbean, particularly in Puerto Rico, the Dominican Republic, Trinidad, Cuba and the Bahamas. It is also found in Central America (Belize, Costa Rica, Guatemala, Honduras, Mexico, Panama) and South America (Columbia, Venezuela).
In the United States, the fly is found in southern Texas and southern Florida.
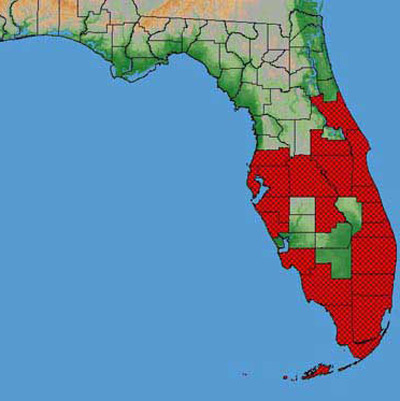
Figure 2. Florida distribution of the papaya fruit fly, Toxotrypana curvicauda Gerstaecker, in 2004. Drawing by G. J. Steck and B. D. Sutton, Division of Plant Industry.
Description (Back to Top)
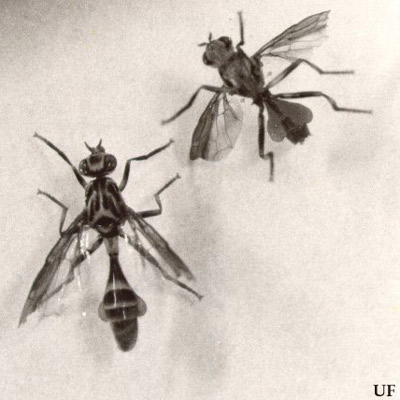
Adult: Commonly mistaken for a vespid wasp due to its size, form, coloration, and behavior, the papaya fruit fly is predominantly yellow marked with black. The female has a very long, slender abdomen with a greatly elongated, curved ovipositor which exceeds the length of its body (body length: 8.5-12.5 mm; ovipositor length: 9-14 mm). The male fly resembles the female with a hairy, but less markedly banded, stalked abdomen and without the ovipositor (body length: 11-13.5 mm; wing width: 8.5-11 mm).

Figure 3. Adult female. Drawing by Division of Plant Industry.
Figure 4. Adult male papaya fruit flies, Toxotrypana curvicauda Gerstaecker, dorsal view (lower left) and ventral view (upper right). Photograph by James L. Nation, University of Florida.
Egg: The egg is long, slender, and yellow with a long cylindrical stalk. The average length is approximately 2.5 mm, and the largest diameter is 0.2 mm.
Larvae: Larvae are white and the typical fruit fly shape (cylindrical-maggot-shape, elongate, with the anterior end narrowed and somewhat recurved ventrally, with anterior mouth hooks, ventral fusiform areas and flattened caudal end). The last instar is large compared to other species at 13-15 mm in length. Other features include the venter with fusiform areas on segments 4 through 11, anterior buccal carinae narrow, long and usually 13 to 15 in number, anterior spiracles nearly straight on dorsal edge but with noticeable depression centrally, with tubules numerous, varying from 22 to 28 and usually with some tubules in a somewhat secondary dorsal row.
Figure 5. Larvae of the papaya fruit fly, Toxotrypana curvicauda Gerstaecker, in papaya. Photograph by Scott Bauer, USDA.
Figure 6. Head and buccal carinae of larva. Drawing by Division of Plant Industry.
The primary diagnostic characters for papaya fruit fly larvae involve the large anterior spiracles, the number of narrow buccal carinae (13 to 15), the lack of prominent tubercles on the caudal end of the larva, and a bifid anal elevation. The anterior spiracles are longer than in most other known fruit fly larvae. The caudal end of the body is particularly distinctive in lacking any prominent tubercles or papillules and has only the spiracular region as a depressed plate, but some indistinct and small tubercles and papillules are present. However, relative to other tephritid larvae, the species has the appearance of a smooth caudal end. The pharyngeal skeleton is particularly distinctive due to the sclerotization along the dorsal margin of the pharyngeal plate.

Figure 7. Anterior spiracles of larva. Drawing by Division of Plant Industry.
Cephalo-pharyngeal skeleton with large convex mouth hook (approx. 2× hypostome length), having a large bulbous lower muscle attachment; hypostomium long, with bulbous subhypostomium; post-hypostomial plates curved dorsally to dorsal bridge sclerotizations; parastomium prominent, pointed; anterior of dorsal bridge with a slightly sclerotized point, more extensive internally towards dorsal wing plate; pharyngeal plate somewhat shorter than dorsal wing plate, both with relatively extensive sclerotizations, especially distinctive along the mid-dorsal margin of the pharyngeal plate beneath median hood.
Figure 8. Cephalo-pharyngeal skeleton of larva. Drawing by Division of Plant Industry.
The sclerotizations of the cephalo-pharyngeal skeleton vary to some extent (see Figure 35 in Phillips 1946), but the prominent sclerotization of the dorsal margin of the pharyngeal plate, the strong central sclerotizations and the large mouth hooks are distinctive for the species. The anterior spiracles usually have a large number of tubules when there is an apparent secondary row of tubules. Illustrations and descriptions of the caudal end of the larva of this species have been incorrect in past works (Berg 1979, Phillips 1946) in not noting the small D1-2 and triangle of L1-3 papillules. Compared to other fruit fly larvae these papillules, as well as the small tubercle for papillule V1, are not readily evident; thus, the larvae appear to have a nondescript smooth caudal end. Careful examination, however, reveals the papillules, tubercles and raised plates as illustrated herein. Published keys to larvae (e.g., Berg 1979) are not compromised, however, since routine identification efforts will not generally make note of the small papillules and tubercles. Larvae examined came from verified samples from Florida in the larval collection of the Florida State Collection of Arthropods.
Caudal end lacking any prominent tubercles or papillules but with several small papillules; the caudal end generally convex, with depressed subquadratic spiracular plate; all papillules not easily seen, with paired dorsal papillules (D1 and D2) angled dorsally, an obtuse triangle of three intermediate papillules (I1-3) ventral to spiracular plate, and a single small V1; all tubercles or raised plates very slightly elevated to maintain an overall impression of a relatively smooth caudal end; L1 papillule not evident; I1 somewhat more prominent than all other papillules; posterior spiracles elongate (approx. 4× to 5× width), with central spiracles nearly straight, dorsal pair slightly angled, and ventral pair angled ventrally; interspiracular processes (hairs) in small tufts with needle-like extensions; spiracular wall thick and internal bars prominent; anal elevation with lobes bifid, rounded.
Figure 9. Caudal end of larva. Drawing by Division of Plant Industry.
Figure 10. Posterior spiracles (left side) of larva. Drawing by Division of Plant Industry.
Figure 11. Anal lobes of larva. Drawing by Division of Plant Industry.
Pupa: The puparia are stout and cylindrical with rounded ends and vary in length from 8.5–12 mm. They are yellow to almost black. The color does not indicate age, as some remain light colored until the adults emerge.
Life Cycle (Back to Top)
The female is capable of producing 100 or more eggs. The female fruit fly oviposits in the green immature fruit by thrusting her ovipositor through the flesh of the fruit. She then deposits a group of 10 or more long, slender eggs in the papaya's central cavity where the young larvae feed on developing seeds and the interior parts of the fruit. As the larvae mature, they eat their way out of the fruit, drop to the ground beneath the plant, and pupate just below the soil surface. Flies emerge in about two to six weeks, depending upon humidity and temperature of the soil.
Eggs are usually laid in small fruit, about two to three inches in diameter, but they may be deposited in smaller or larger fruit. However, unripe papaya juice is fatal to the larvae so the fruit must be ripe before the larvae begin to eat their way out of the inner cavity. Eggs hatch approximately 12 days after oviposition and larval development in the fruit lasts about 15 to 16 days.
Damage (Back to Top)
Fruit infected with papaya fruit fly larvae will turn yellow and drop from the tree prematurely. Damage levels in Florida fluctuate between 2 and 30% of fruits infested during the spring-summer season.
Management (Back to Top)
Prevention of egg-laying is the key to controlling papaya fruit fly. It is necessary to kill the adult female before she deposits eggs in the fruit.
Florida Crop/Pest Management Profile: Papaya
Bagging can be an effective control measure for the fruit fly in small plantings (one to 25 plants or less than 1/10 hectare). Bagging should begin when the fruit is small, shortly after the flowers have fallen off. Each fruit should be enclosed in a paper bag or rolled tube of newspaper and tied around the stem. This method can be very practical and successful if enough labor is available. Attention to covering new fruit and increasing the covering as the fruits increase in size is necessary.
Sanitation is also important in the control of the papaya fruit fly. All dropped and prematurely ripe fruit, as well as infested young fruit, must be destroyed in order to prevent the larvae from developing into adults.
Very little research has been done on the biological control of papaya fruit fly, considering its economic importance. One parasitic wasp, in particular, Doryctobracon toxotrypanae Marsh (Hymenoptera: Braconidae), from southern Mexico and Costa Rica, may have potential for control.
Pheromone lures and traps are critical for monitoring fruit fly populations, measuring distribution, and acquiring control or eradication. Research in Miami and Gainesville, Florida has resulted in the development of a pheromone lure and functional trap for this species. Studies are presently being performed to test female pheromone lures with the addition of host odor chemicals in order to increase the attraction of flies and to improve its performance in competition with males.
Other possible control measures have been suggested for future research, such as papaya fruit without seeds. Due to the strong dependence of larval feeding on the seeds as an early food source, a seedless papaya variety could be explored. For similar reasons, thicker pulp could possibly provide enough resistance to hinder the ovipositor from successfully depositing eggs. Another suggestion has been to look for varieties of papaya with higher levels of chemicals that are toxic to the larvae.
Selected References (Back to Top)
- Benjamin FH. 1934. Descriptions of some native trypetid flies with notes on their habits. U.S. Department of Agriculture Technical Bulletin 401: 1-95.
- Berg GH. 1979. Pictorial key to fruit fly larvae of the family Tephritidae. San Salvador: Organ. Internac. Region. Sanidad Agropec. 36 pp.
- Castrejon-Ayala F, Camino-Lavin M. 1991. New host plant record for Toxotrypana curvicauda (Diptera: Tephritidae). Florida Entomologist 74: 466.
- Greene CT. 1929. Characters of the larvae and pupae of certain fruit flies. Journal of Agricultural Research 38: 489-504.
- Ibrahim RB. 1980. Fruit Flies of Florida (Diptera: Tephritidae). Ph. D. Dissertation: University of Florida. Toxotrypana curvicauda Gerstaecker. p. 188-190.
- Knab F, Yothers WW. 1914. Papaya fruit fly. Journal of Agricultural Research 2: 447-453.
- Landolt PJ. 1994. Fruit of Morrenia odorata (Asclepiadaceae) as a host for the papaya fruit fly, Toxotrypana curvicauda (Diptera: Tephritidae). Florida Entomologist 77: 287-288.
- Landolt PJ. 1990. Behavior of the papaya fruit fly (Diptera: Tephritidae): host finding and oviposition. Environmental Entomology 19: 1305-1310.
- Landolt PJ. 1984. Behavior of the papaya fruit fly, Toxotrypana curvicauda Gerstaecker, (Diptera: Tephritidae). Folia Entomologica Mexicana 61: 215-224.
- Landolt PJ, Heath RR. 1996. Development of phermone-based trapping systems for monitoring and controlling tephritid fruit flies in Florida. pp. 197-207. In Rosen D, Bennett FD, Capinera JL. (editors)., Pest Management in the Subtropics. Intercept Limited, United Kingdom.
- Mason AC. 1922. Biology of the papaya fruit fly, Toxotrypana curvicauda, in Florida. U.S. Department of Agriculture Technical Bulletin 1081. 10 pp.
- Peña JE. 1988. Effectiveness of pesticides against two tropical fruit pests. Proceedings of the Florida State Horticultural Society 101: 249-251.
- Peña JE, Howard DF, Litz RE. 1986. Feeding behavior of Toxotrypana curvicauda (Diptera: Tephritidae) on young papaya seeds. Florida Entomologist 69: 427-428.
- Phillips VT. 1946. The biology and identification of trypetid larvae (Diptera: Trypetidae). Memoirs of the American Entomological Society 12: 1-161.
- White IM, Elson-Harris MM. 1994. Fruit Flies of Economic Significance: Their Identification and Bionomics. CAB International. Oxon, UK. 601 pp.
- Wolfenbarger DO, Walker SD. 1974. Two major pest problems of papayas. Proceedings of the Florida State Horticultural Society 87: 384-385.