common name: melon fly
scientific name: Bactrocera cucurbitae (Coquillett) (Insecta: Diptera: Tephritidae)
Introduction - Synonyms - Distribution - Description - Life History - Damage - Hosts - Management - Selected References
Introduction (Back to Top)
Within its range, the melon fly, Bactrocera cucurbitae (Coquillett), is one of the most important pests with which vegetable growers have to contend. Although found in Hawaii, it is not present in the continental United States.
Figure 1. Adult melon fly, Bactrocera cucurbitae (Coquillett). Photograph by Scott, Bauer, USDA.
Synonyms (Back to Top)
Chaetodacus cucurbitae (Coquillett)
Dacus cucurbitae Coquillett
Strumeta cucurbitae (Coquillett)
Zeugodacus cucurbitae (Coquillett)
Distribution (Back to Top)
The melon fly is well distributed over most of India, which is considered its native home, and throughout most of southeastern Asia. It was introduced into the Hawaiian Islands from Japan about 1895. By 1897, when it was first observed, it was already a serious pest. Other populations are reported as follows (CABI 2003):
Africa: Cameroon, Cote d'Ivoire, Egypt, Gambia, Kenya, Mali, Maritius, Reunion, Seychelles, Somalia, Tanzania
Asia: Afghanistan, Bangladesh, Brunai, Cambodia, China (numerous provinces), Christmas Island, East Timor, India (numerous states), Indonesia (numerous islands), Iran, Laos, Malaysia, Myanmar, Nepal, Oman, Pakistan, Philippines, Singapore, Sri Lanka, Taiwan, Thailand, United Arab Emirates, Vietnam
North America: United States: established in Hawaii, periodic interceptions in other states
Oceania: Guam, Kirbali, Nauru, Northern Mariana Islands, Papua New Guinea, Solomon Islands
It is not established in the continental United States, although it often is intercepted at ports. As recently as August 2010, several flies were discovered in Kern County, California. Quarantine and eradication measures were quickly enacted and these measures are continuing as of this publication date (NAPPO 2010).
Description (Back to Top)
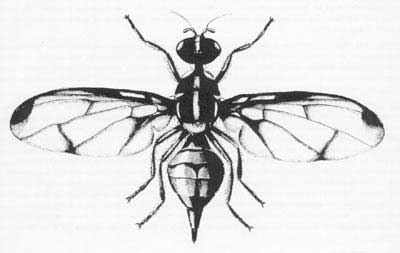
Adult: The adult fly is 6 to 8 mm in length. Distinctive characteristics of the adult are the wing pattern, long third antennal segment, the dorsum of the thorax reddish yellow with light yellow markings and without black markings, and the head yellowish with black spots.
Figure 2. Adult female melon fly, Bactrocera cucurbitae (Coquillett). Photograph by Jack Clark, University of California.
Figure 3. Adult wing venation. Drawing by Division of Plant Industry.
Egg: The egg is pure white, about 2 mm long, elliptical, nearly flat on the ventral surface, more convex on the dorsal. Eggs often are somewhat curved.
Larva: The larva has three instars. The larva is white, except when its appearance is altered by the color of the food within the alimentary canal. The larva has a typical fruit fly larval shape - cylindrical-maggot shape, elongate, anterior end narrowed and somewhat curved ventrally, with anterior mouth hooks, ventral fusiform areas and flattened caudal end. The last instar larva ranges from 7.5–11.8 mm in length.
The larva of the melon fly is particularly distinctive in having a dark sclerotized horizontal line below the spiracular region on the caudal end, with a curved ridge on each side of it. No other known fruit fly larva has this combination of characters, plus other features of the anterior spiracles and cephalo-pharyngeal skeleton (Berg 1979, Chu 1949, Green 1929, Hardy 1949, Phillips 1946, Pruitt 1953).
A more technical description of the larva follows:
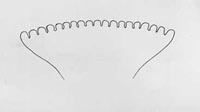
Venter with fusiform areas on segments 2 through 11 (2 through 4 are weakly developed); anterior buccal carinae usually 18 to 20 in number; anterior spiracles slightly convex in lateral view, with tubules averaging 18 to 20 in number and relatively small.
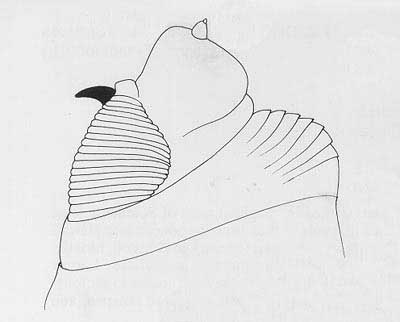
Figure 4. Head and buccal carinae. Drawing by Division of Plant Industry.
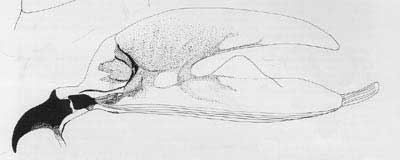
Figure 5. Anterior spiracle. Drawing by Division of Plant Industry.
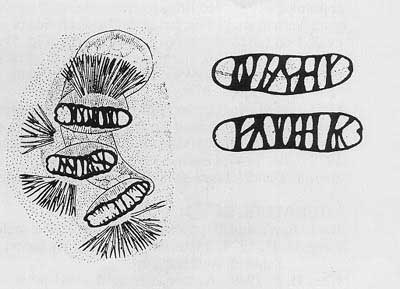
Cephalo-pharyngeal skeleton with large, sharply pointed convex mouth hook each side, with prominent dorsal lobe, and each hook about 3× hypostome length; hypostomium with prominent, semi-rounded subhypostomium; post-hypostomial plates curved to dorsal bridge, fused with sclerotized rays of central area of dorsal wing plate; parastomium prominent; dorsal wing plate with posterior ray split; dorsal bridge relatively unsclerotized except on posterior curve to pharyngeal plate; a prominent hood on pharyngeal plate and several striae ventrally.
Figure 6. Cephalo-pharyngeal skeleton. Drawing by Division of Plant Industry.
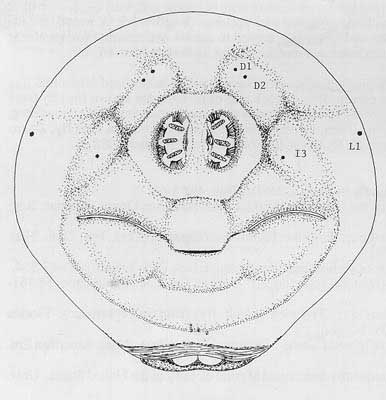
Caudal end with paired dorsal papillules (D1 and D2) diagonally dorsad each spiracular plate; intermediate papillules as a curved ridge to each side, with a prominent central dark line below spiracles about midway between spiracles and anal lobes, but with I3 to each side of spiracles; L1 on median edge of caudal end; ventral papillules not evident on raised plates; posterior spiracles as three elongated oval openings (length = 3 to 3.5× width) on each kidney-shaped spiracular plate, with dorsal spiracles and lower pair angled to caudal end center; interspiracular processes (hairs) numerous, at 4 sites on each plate, tips bifurcate; anal lobes entire and small.
Figure 7. Caudal end of larva. Drawing by Division of Plant Industry.
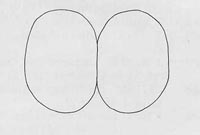
Figure 8. Posterior spiracles. Drawing by Division of Plant Industry.
Figure 9. Anal lobes. Drawing by Division of Plant Industry.
Pupa: The puparium is about 5 to 6 mm in length and varies in color from dull red or brownish yellow to dull white, depending on the host.
Life History (Back to Top)
Development from egg to adult under summer conditions requires from 12 to 28 days, according to the individual and to host and weather conditions. The developmental periods may be extended considerably by cool weather. The length of the stages in the Philippine Islands, at an average temperature of 86°F, was 1.73 days for eggs, 4 to 9 days for larvae, and 7 to 11 days for pupae. Laboratory tests in Hawaii prolonged the egg stage to 11 days, the larval stage to 30 days, and the pupal stage to 51 days, by exposing specimens to low temperatures. In the Philippines, the preoviposition period lasted 7 to 26 days and the oviposition period 39 to 95 days.
A single hardy female may lay as many as 1,000 eggs. Eggs generally are laid in young fruit, although they are laid also in succulent stems of many host plants, in cavities made with the aid of a sharp ovipositor. Only ripe fruit of some hosts are attacked. Pupation normally occurs in the soil, usually beneath the host, at a depth of up to 2 inches. Adults may live more than a year. Adults feed primarily upon juices of host plants, nectar, and honeydew secreted by various kinds of insects. There may be as many as eight to 10 generations a year.
Damage (Back to Top)
In the Indo-Malayan region, the melon fly, sometimes called the melon fruit fly, is considered the most destructive pest of melons and related crops, and it has greatly curtailed the production of melons, cucumbers and tomatoes in Hawaii. The establishment of this fly in areas similar to Florida indicates that this species could become a serious pest of cucurbits and other truck crops, and possibly of some fruit crops, if it were introduced into Florida.
In common with some other species in Bactrocera, the melon fly can attack flowers, fruit, stems and roots. In Hawaii, pumpkin and squash have been heavily attacked even before the fruit had set, with eggs laid into unopened male and female flowers, with larvae successfully developing in the taproots, stems and leaf stalks.
Hosts (Back to Top)
More than 125 species of plants, including cucurbits, tomatoes, and many other vegetables, have been recorded as hosts of the melon fly.
Preferred hosts include: cantaloupe, cowpea, cucumber, gourd, pumpkin, squash, string bean, tomato and watermelon. However, White and Elson-Harris (1994) state that many of these records may have been based on casual observations of adults resting on plants or caught in traps set in non-host trees.
Occasional hosts include: eggplant, fig, mango, orange, papaya and peach. Wild hosts include: balsam apple; Chinese cucumber, Momordica spp.; colocynth; two genera of cucurbits—Sicyos sp.; Cucumis trigonus; Diplocyclos palmatus; and passion-flower, Passiflora spp.
Management (Back to Top)
As Bactrocera cucurbitae (Coquillett) is not found in the continental United States there are no specific management recommendations for its control in Florida. In the Solomon Islands it was subjected to an eradication campaign using a combination of bait spraying and male annihilation traps. In the Ryukyu Islands it was eradicated from some islands using the sterile insect technique. The larval parasitoid Psyttalia fletcheri (Silvestri) and the egg parasitoid Fopius arisanus (Sonan) were introduced into Hawaii to parasitize Bactrocera cucurbitae (Bautista et al. 2004).
Figure 10. Adult Psyttalia fletcheri (Silvestri), a parasitoid of the melon fly Bactrocera cucurbitae (Coquillett). Photograph by Scott, Bauer, USDA.
Selected References (Back to Top)
- Anonymous. 1959. Insects not known to occur in the United States. Cooperative Economic Insect Report 9 (19): 343-368. Melon fly (Dacus cucurbitae (Coq.)), pp. 367-368.
- Back EA, Pemberton CE. 1917. The melon fly in Hawaii. U.S. Department of Agriculture Bulletin 491: 1-64.
- Berg GH. 1979. Pictorial key to fruit fly larvae of the family Tephritidae. San Salvador: Organ. Internac. Reg. Sanidad. Agropec. 36 pp.
- Cáceres C, McInnis D, Shelly T, Jang E, Robinson A, Hendrichs J. 2007. Quality management systems for fruit fly (Diptera: Tephritidae) sterile insect technique. Florida Entomologist 90: 1-9.
- Chu HF. 1949. A classification of some larvae and puparia of the Tephritidae (Diptera). Cont. Inst. Zool., Natl. Acad. Peiping (Beijing) 5: 93-138.
- Green CT. 1929. Characters of the larvae and pupae of certain fruit flies. Journal of Agricultural Research (Washington) 38: 489-504.
- Hardy DE. 1949. Studies in Hawaiian fruit flies (Diptera, Tephritidae). Proceedings of the Entomological Society of Washington 51: 181-205.
- Heppner JB. 1988. Larvae of fruit flies IV. Dacus dorsalis (Oriental fruit fly) (Diptera: Tephritidae). Florida Department of Agriculture and Consumer Services, Division of Plant Industry Entomology Circular 303: 1-2.
- Foote RK, Blanc FL. 1963. The fruit flies or Tephritidae of California. Bulletin of the California Insect Survey 7: 1-117.
- Phillips VT. 1946. The biology and identification of trypetid larvae (Diptera: Trypetidae). Memoirs of the American Entomological Society 12: 1-161.
- Pruitt JH. 1953. Identification of Fruit Fly Larvae Frequently Intercepted at Ports of Entry of the United States. University of Florida (Gainesville), M.S. thesis. 69 pp.
- Shelly TE, Pahio E, Edu J. (2004). Synergistic and inhibitory interactions between methyl eugenol and cue lure influence trap catch of male fruit flies, Bactrocera dorsalis (Hendel) and B. cucurbitae (Diptera: Tephritidae). Florida Entomologist 87: 481-486.
- USDA, Survey and Detection Operations, Plant Pest Control Division, Agriculture Research Service. Anonymous. 1963. The melon fly. Pamphlet 581. 4 pp.
- White IM, Elson-Harris MM. 1994. Fruit Flies of Economic Significance: Their Identification and Bionomics. CAB International. Oxon, UK. 601 pp.