common name: atala butterfly, atala hairstreak, coontie hairstreak
scientific name: Eumaeus atala Poey 1832 (Insecta: Lepidoptera: Lycaenidae)
Introduction - Distribution - Description - Life Cycle - Host Plants - Natural Enemies and Diseases - Management - Acknowledgments - Selected References
Introduction (Back to Top)
The Atala butterfly, Eumaeus atala Poey, is the largest and most iridescent hairstreak in southeastern Florida. Due to the decline in the abundance and distribution of its host plant caused by overharvesting the root for starch production by early settlers, the Atala butterfly was thought to be extinct from 1937 until 1959 (Klots 1951, Rawson 1961). Although still considered rare with limited distribution, it is now found in local colonies where its host plant, coontie (Zamia integrifolia Linnaeus. f.), is used in butterfly gardens or as an ornamental plant in landscapes. In fact, the butterfly caterpillars are a source of consternation for many nurseries, botanical gardens, and some homeowners because of the foliar damage.
Figure 1. Eumaeus atala Poey, pinned and spread adult female. Photograph by Donald W. Hall, University of Florida.
Like a number of other butterfly genera names, Eumaeus originates from Greek mythology. Eumaeus was the swine herder and friend of Odysseus, hero of the Trojan War in Homer’s Iliad and Odyssey. The specific epithet atala is from Atala, the Indian princess from Chateaubriand’s novel Atala/René (Opler and Krizek 1984).
Distribution (Back to Top)
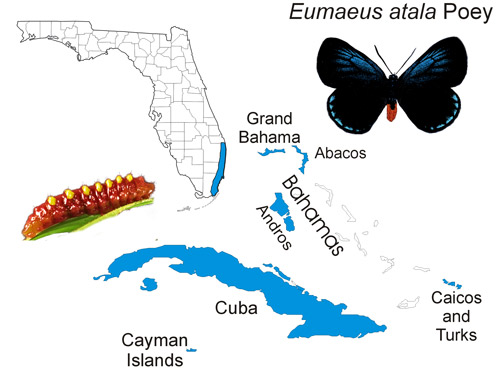
The Atala butterfly occurs naturally in subtropical Palm Beach, Broward and Miami-Dade counties in southeastern Florida, although some ephemeral (short-lived) colonies have been recently introduced into Martin, Monroe and Collier counties. The butterfly is also found in the Bahamas (at least Grand Bahama, Great Abaco, and Andros) (Smith et al. 1994), Turks and Caicos, Cayman Islands, and Cuba, in areas where the host plant occurs (Minno and Emmel 1993, Opler and Krizek 1984) (Figure 2). Smith et al. (1994) reported that the Atala butterfly is widespread in Cuba including the Isle of Pines (now known as: Isla de la Juventud “the Island of Youth”), but most records are from the Havana area.
Figure 2. Eumaeus atala Poey, distribution map. Map based on Smith et al. (1994) and Minno et al. (2005). Map by Donald W. Hall, University of Florida.
Description (Back to Top)
Adults: The adult Atala butterfly forewing cord length ranges from under 2 cm to 2.5 to 2.7 cm, which is both seasonally and genetically driven; adult size is partly determined by the quality and availability of its host plant, which the larvae (caterpillars) eat. The wing cord length is measured from the cell that connects the wing to the thorax to the tip of the fore wing (Figure 3).
Figure 3. Eumaeus atala Poey, wing-cord length method of wing measurement. Photograph by Sandy Koi, University of Florida.
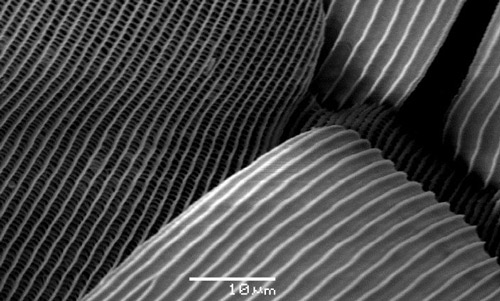
The outer surface of the folded wings of both males and females is deep black, bordered around the edges with three curved rows of ultramarine spots. These spots are iridescent due to constructive interference of selected wavelengths of light by microscopic structural formations in the scales of the wings (Wilts et al. 2009) (Figure 4). Functions of iridescence in animals (including insects) have been reviewed by Doucet and Meadows (2009). Two of the functions suggested for iridescence are defense (aposematism) and mate recognition. The iridescent spots on Atala butterflies vary slightly in size and shape between every individual (Koi 2013, 2015). The ultramarine color also rings the eyes, and may be found on the legs and thorax as well.
Figure 4. Eumaeus atala Poey, microstructure of iridescent scales. Photograph by Sandy Koi, University of Florida.
The underside of the hindwing also displays a large bright red spot on the mid-caudal area (Figure 3), and the abdomen is red-orange. These red scales are not formed by the same microscopic structural forms as the ultramarine spots and are not reflective.
In male Atala butterflies, the upper side of the open wings displays bright iridescent Caribbean blue or teal green scales that cover most of the upper wing and some of the lower hind wing (Figure 5). The color is partially genetically determined, and is also associated with seasonal humidity, daylight length, and temperatures; blue scales are more common in the warmer summer months and green scales are more prominent during the cooler winter months (Koi 2013, 2015). Males are generally slightly smaller than females.
Figure 5. Eumaeus atala Poey adult males showing teal green (top) and Caribbean blue (bottom) iridescence on forewings. Photograph by Sandy Koi, University of Florida.
Females display bright iridescent royal blue color on part of the upper surface of the forewings (Figure 6).
Figure 6. Eumaeus atala Poey adult female showing royal blue streak on forewings. Photograph by Sandy Koi, University of Florida.
Eggs: The highly sculpted cream-colored eggs are laid in clusters of up to 60 or more, usually on the new leaf tips of the host plants. Eggs measure an average of about 1 mm, up to 1.25 mm (Koi 2013, 2015).
Females often cover the eggs with bright red-orange scales from their anal tufts (Figure 7). According to Rothschild (1992), the conspicuous scales are considered to provide an aposematic (warning) signal to potential predators. Atala butterflies store much of their cycasin (methylazoxymethanol glucoside), a protective chemical compound sequestered from their larval host plant, in their wings, but also a significant amount is found in the eggs (Schneider et al 2002). However, Schneider et al. (2002) were unable to find cycasin in the wing scales. Cycasin is a toxin and carcinogen (Rothschild et al. 1986, Nash et al. 1992) and is classified as a β-glycoside of methylazoxymethanol (Rothschild et al 1986, Nash et al. 1992, Yagi 2004).
Figure 7. Eumaeus atala Poey eggs with aposematic scales from anal tuft of female. Photograph by Hank Poor.
Eggs are glued to the surface of the host plant by proteins secreted by the female and are clear on the underside, allowing the yolk or embryos to be seen (Koi 2013, 2015). In late winter, when there is less new growth on the plants, females will sometimes oviposit on the reproductive cones of the coontie plants.
Larvae: Newly hatched larvae (caterpillars) of the Atala butterfly are tiny (0.5 to 1 mm), pale and flesh-colored (Figure 8), but develop into bright red caterpillars with two rows of lemon-yellow spots displayed dorsally within a day or two. The bright red coloration with yellow spots is retained throughout larval development (Figure 9). The bright aposematic coloration is a warning that they are toxic to potential predators. Full-grown larvae are approximately 2.5 cm (about one inch) in length.
Figure 8. Eumaeus atala Poey newly hatched larvae. Photograph by Sandy Koi, University of Florida.
Figure 9. Eumaeus atala Poey mature larva. Photograph by Jerry Butler, University of Florida.
Photographs of larval exuviae (Figure 10) illustrate the relative sizes of the instars (Koi 2013, 2015).
Figure 10. Eumaeus atala Poey exuviae of progressive larval instars for size comparison. Photograph by Sandy Koi, University of Florida.
Pupae: Prepupae begin changing color on the ends (Figure 11). Warm-season pupae are a golden brown color with black spots; cool season pupae are darker brown with black spots (Koi 2013, 2015).
Figure 11. Eumaeus atala Poey progression from prepupa to pupa. A) and B) Pre-pupa, C) Newly-molted pupa, D) More mature pupa that has not yet darkened completely. Photographs by Sandy Koi, University of Florida.
Pupae are supported by a very fine silk girdle around the thorax. The cuticle is often covered with bitter-tasting droplets of unknown origin (Minno and Emmel 1993). Nash et al. (1992) described these as “bitter” pyrrolizidine alkaloids from the hostplant and are probably utilized in a protective role by the pupa.
Figure 12. Healthy, eighteen-day-old Eumaeus atala Poey pupa that will eclose within a day or two. Note black wing pads, reddish abdomen, and bitter-tasting droplets. Photograph by Sandy Koi, University of Florida.
Life Cycle (Back to Top)
Adults: The Atala butterfly is multivoltine (with year-round breeding), and different colonies may temporarily die out while others are erupting (growing large populations). Typically, there are reduced population counts in March and December and higher population counts in June and January (Koi 2013).
Both males and females are relatively sedentary if the nectar sources and host plants are available. Adults have a slow moth-like flight pattern, although males in particular can fly very fast and erratically, performing aerial displays to attract females and challenge male rivals.
Adult Atala butterflies may live as long as three weeks or more in the wild and have been shown to live two to nearly three months in colonies with ideal nectar and host plant resources (Koi 2013, 2015).
Males often perch on the upper side of leaves in order to scan the surroundings for females (Koi 2013) or competitor males, while females often hang upside-down under leaves. Males have hair brushes or pencils at the tips of their abdomens. They court females by hovering in front of them while fanning the hair pencils with their wings (Opler and Krizek 1984, Scott 1986) - presumably wafting a courtship pheromone from the pencils to the females’ antennae (Schneider 2001). Schneider et al. (2002) suggested that the highly visible yellow hair pencils may also serve as an optical or even a mechanical courtship signal.
Figure 13. Eumaeus atala Poey males display anal hair pencils when courting females. Photograph by Sandy Koi, University of Florida.
Female Atala butterflies are not aggressive to other females, often laying eggs side by side on the host plant. Adults are easily approached and sit undisturbed - their behavior reflecting their protection from the toxic cycasin sequestered from their larval host plants. Adults are most active in the early morning and late afternoon.
Atalas of both sexes mate multiple times and because of the extended lifespan there may be intergenerational mating as well (Koi 2013, 2015). This behavior promotes greater genetic diversity, which helps explain why some isolated colonies may persist for years in fragmented habitats.
Larvae: Newly hatched caterpillars line up side-by-side for the first few days of life and skeletonize the tough cuticle of the leaves. They are gregarious until the fourth or fifth instar.
Larvae have three to five instars (molts). The color of the different instars does not change as it does with some other butterfly species. Atala larvae are unusual in their ability to pupate as early as the third instar if environmental conditions are unfavorable, resulting in smaller than average adults (Koi 2013, 2015). Larvae are able to survive on female coontie cones as well as those of other cycad species.
Larvae begin to search for a pupation site at about the tenth day of the last instar (Koi 2013), and if they have moved apart to feed, will usually regroup and transfer en masse to the chosen location. Larvae usually move to the pupation site in the late evening and may crawl a considerable distance away (Koi 2013, 2015). The larvae release silk that anchors them to the base of their pupation site and to each other. This additional connection may act as protection against being dislodged in high winds or tropical storms.
Pupae: Pupae often form large, extensive silk mats when they gather together as larvae to pupate in a cluster, usually on the host plant or nearby (Figure 13). Most pupae are found close to the ground, but some have been observed above eye level in trees. Late instar larvae of different generations often gather for pupation at the same sites - suggesting the possibility of an aggregation pheromone.
Figure 14. Eumaeus atala Poey, cluster of newly formed pupae. Photograph by Sandy Koi, University of Florida.
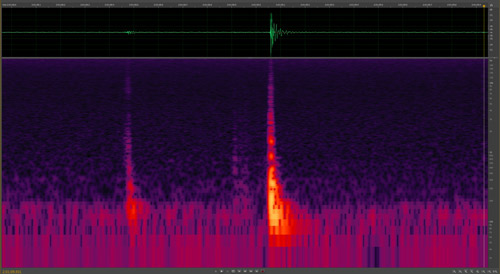
Many species of Lycaenid butterfly pupae stridulate (produce sounds) (Hinton 1948, Downey 1966), but the functions have not been fully explored. Suggested functions include its use as a predator deterrent, defense against parasitoids, a signal indicating imminent eclosure to conspecifics, communication with symbiotic ants for those species associated with ants, and to communicate conspecific aggregation formation (Downey 1966). Atala pupae were known to stridulate (Rothschild 1986, 1992), but until recently the frequency and range was unknown (Koi 2013). At ten days old, Atala pupate produce short bursts of sound (Koi 2013), which may indicate to conspecifics that eclosure is imminent, possibly to synchronize emergence for mating (Downey 1966, Hoegh-Guldberg 1972) (Figure 15). The sound produced by ten-day old pupae is not audible to human ears (Koi 2013). No sounds were detected from younger pupae or larvae under different experimental manipulations in a sound chamber (Koi and Mankin unpublished).
Figure 15. Eumaeus atala Poey sonogram of pupal stridulation – amplified at bottom (red and yellow peaks) for better visualization. Both sounds were made at a frequency between 100-500 Hz and lasted for 0.07 seconds. Photograph by Jim Schlachta.
Host Plants (Back to Top)
The Atala butterfly’s only native host plant, coontie (Figure 16), is also the only cycad native to North America, and until relatively recently was the only host plant available. Coontie has gone through numerous taxonomic name changes (Ward 2015). We have followed the Flora of North America classification and used the name Zamia integrifolia L. f., but it has also been known as Zamia pumila and Zamia floridana. Currently, both the USDA Plants Database and Atlas of Florida Vascular Plants (see Selected References section) treat Zamia integrifolia as a synonym of Zamia pumila. See the Wikipedia entry and Ward (2015) for a discussion of the naming controversy and relevant references.
An interesting side note to the name integrifolia is that the name was assigned not by the famous taxonomist Carl Linnaeus but rather by his son. The “f.” in the author name stands for the Latin “filius” which means “the son”. (See: https://en.wikipedia.org/wiki/Carl_Linnaeus_the_Younger).
Figure 16. Healthy coontie plants (Zamia integrifolia) growing in an urban garden. Photograph by Sandy Koi, University of Florida.
In a natural setting, such as the historic pine rocklands of south Florida, coontie plants grow in ever-expanding clusters near each other and are generally small because of the limestone substrate and lack of nutrients. This growth habit benefits the adults and caterpillars, because the females do not have to fly far to find the host plants and the caterpillars do not have to disperse far in search of new foliage.
Coontie has a mutualistic relationship with the Atala butterfly. In return for the plants providing food for the caterpillars, the abundant fecal droppings of the caterpillars provide fertilizer for the plant (Koi 2013) and enrich the nutrient-poor pine rocklands soil.
Cycads are dioecious (individual plants have cones of only one sex). Cones on female plants are larger and thicker than those on male plants (Minno et al. 2005). The plants also have specialist pollinators, small beetles (Rhopalotria slossoni Schaffer (Curculionidae) and Pharaxonotha zamiae Blake (Erotylidae)) that live inside the male cones. The beetles emerge seasonally when environmental conditions are favorable and carry pollen from the male plants to the female plants.
The toxins in the cycad hostplants are water-soluble and thus it was possible for the original natives and early settlers to carefully process the root as the primary starch source, described in detail by the early Spanish explorers (Laudonièrre 1975). However, historical records also indicated that run-off water from the processing method could and did poison livestock and domestic animals (Hooper 1978, Bowers and Larin 1989). The native colonies of wild coontie were nearly extirpated by the starch industry during the early 1900s (Coile 2000), but residential and commercial plantings since have greatly helped in the plants’ recovery (Dehgan 2002, Haynes 2000, Coile and Garland 2003) and bolstered the Atala colonies in the process (Koi 2013, 2015, Ramírez-Restrepo et al. In review).
More than thirty species of exotic cycads have been planted in the tropical gardens in southeastern Florida as ornamentals during the last eighty years, and Atala butterfly females will oviposit on all of them (Hammer 1996, Koi 2013). Cycads are considered globally imperiled and all contain toxic cocktails composed of the same basic chemicals in different proportions, including the azoxyglucosides, macrozamin, cycasin, and other toxins (Yagi 2004). Atala larvae will develop on all cycads but may not thrive on some of the exotic species as well as they do on native coontie. The primary toxin in coontie is cycasin, heavily concentrated in the new coontie growth favored by the Atala larvae.
Atala larvae sequester the chemicals (particularly cycasin) in their tissues, and the toxins are passed on to pupae, adults, and eggs of the next generation to help protect them from potential predators (Rothschild et al. 1986). Bowers and Larin (1989) stated that both cycasin and adult Atala butterflies are a deterrent to the carpenter ant, Camponotus abdominalis floridanus (Buckley). The spermatophores also contain cycasin and may serve as a nuptial gift from males when passed to the female during copulation (Schneider et al. 2002).
The conspicuous colors of the larvae, pupae, and adults demonstrate a perfect example of aposematic coloration. Like other chemically protected animals, the bright colors advertise toxicity and the aggregation behavior of the Atala larvae, pupae and adults helps reinforce this message. A naïve predator may try to eat one of the insects, but seldom tries a second time (assuming they survive the first try).
In a recent experiment, larvae and adults were given a choice between native coontie and a non-native cycad host, Zamia vazquezii D.W.Stev., Sabato & De Luca (Zamiaceae), which is frequently used in southeast Florida ornamental gardens. Larvae and adults both showed a strong preference for the native host compared to the non-native cycad, and the larvae survived better and lived longer on native coontie (Koi 2013).
Large aggregations of larvae are capable of completely defoliating a mature coontie plant, although a healthy, established plant usually recovers within a growing season. Defoliation of plants may be an economic concern if the plants are used in an ornamental garden, commercial nursery or as a landscaping plant, especially if they are rare and exotic cycad species (Figure 16).
Figure 17. Zamia erosa O.F.Cook & G.N.Collins (Zamiaceae), a valuable exotic cycad that has been completely defoliated by Atala larvae in a botanical garden. Photograph by Sandy Koi, University of Florida.
Nectar: A variety of flowers are used as nectar sources (Culbert 1994 and 1995, Koi 2008, Landolt 1984). Because Atala butterflies have a short proboscis, they need flowers with short corollas, although they have been observed climbing head-first into large deep flowers. White-flowered plants seem to attract them more than other colors, and all palm tree inflorescences are particularly attractive. Nectar sources such as wild coffee (Psychotria nervosa Sw. (Rubiaceae)), porterweed (Stachytarpheta jamaicensis (L.) Vahl (Verbenaceae)), beautyberry (Callicarpa americana L. (Lamiaceae)), indigoberry (Randia aculeate L. (Rubiaceae)) and common weeds such as Spanish needles, now known as Romerillo or Beggarticks, (Bidens alba (L.) DC. (Asteraceae)) are utilized readily by the Atala butterflies. Common flowering trees in south Florida such as dahoon holly (Ilex cassine L. (Aquifoliaceae)), sweet almond (Aloysia virgata (Ruiz & Pav.) Juss. (Verbenaceae)), blackbead (Pithecellobium spp. (Fabaceae)), Florida fiddlewood (Citharexylum spinosum L. (Verbenaceae)) and golden dewdrops (Duranta erecta L. (Verbenaceae)) are also used.
Natural Enemies and Diseases (Back to Top)
Parasitoids
Although most butterflies are associated with a parasitoid, there has been no documentation or evidence of parasitoids in the Atala, or any of the other Eumaeus species.
Predators
Few predators have been documented attacking Atala butterflies. A number of non-native and exotic ants including Pharaoh ants (Monomorium pharaonis L. (Formicidae)), Camponotus species (Formicidae), bigheaded ants (Pheidole megacephala Fabricius (Formicidae)) and fire ants (Solenopsis invicta Buren (Formicidae)) are able to avoid the protective scales and have been observed consuming eggs (Smith 2002). The Mexican twig ant (Pseudomyrmex gracilis Fabricius (Formicidae)) is known to attack Atala larvae and many other lepidopteran caterpillars. Ants may preferentially attack newly hatched larvae that have not yet ingested enough cycasin from the host plants to be chemically protected.
Assassin and ambush bugs (Reduviidae) attack Atala larvae and appear to be able to tolerate or detoxify the protective chemicals. There have been unconfirmed reports of starlings, peacocks, and other non-native birds attacking caterpillars, but it is not known if the birds survived the apparent meal. Bowers and Farley (1990) reported that gray jays (Perisoreus canadensis L. (Corvidae)) rejected Atala butterflies. Spiders have been observed cutting Atala butterflies free from their webs (personal observation).
Many local reports indicate that both native and non-native anoles, curlytail lizards (Leiocephalus carinatus armouri Barbour & Shreve (Leiocephalidae)), Cuban tree frogs (Osteopilus septentrionalis (A.M.C. Duméril & Bibron) (Hylidae)) and brown basilisks (Basiliscus vittatus Wiegmann (Corytophanidae))attack Atala larvae as well as newly-eclosed (and therefore vulnerable) adults. Yellow jackets wasps (Vespidae) have been observed attacking adults.
In addition to predation by other species, Atala caterpillars may be cannibalistic on conspecific larvae and pupae in the brood – particularly in confinement (Baggett 1982). The effect of cannibalism of immatures on wild Atala populations is not known. Cannibalism is not uncommon among insects and is a particularly common behavior of lycaenid larvae (Richardson et al. 2010). Cannibalism may be more prevalent in areas with limited resources (insufficient number or quality of host plant), adverse environmental conditions (such as drought, fungal growth, changes in daylight periods or temperature), or increased population density, as well as unknown factors. For a discussion of cannibalism in normally non-cannibalistic insects, see Richardson et al. (2010).
Pathogens
Like other Lepidoptera, Atala butterflies are susceptible to a variety of pathogens throughout their development (Figures 18). Viral infections occasionally occur in laboratory colonies and in nature. In laboratory colonies, viruses are extremely contagious, and care must be taken to isolate healthy larvae from diseased larvae and their contaminated food, feces, and rearing containers.
Figure 18. Eumaeus atala Poey larva exhibiting pose typical of nuclear polyhedrosis virus (NPV) infection. Photograph by Mickey Wheeler.
Figure 19. Eumaeus atala Poey pupae infected with Paeciliomyces sp. fungus. Note the blackened abdomens. Photograph by Sandy Koi, University of Florida.
Developmental Abnormalities
Developmental abnormalities, abnormal molting between instars, and eclosion failure (inability of the adult to successfully emerge from the pupa) may result from a variety of causes and are particularly common in laboratory colonies (Figures 19 to 22). Often it is impossible to determine the cause of the abnormality.
Figure 20. Abnormally formed Eumaeus atala Poey pupae. The black color is due to secondary bacterial growth. Photograph by Sandy Koi, University of Florida.
Figure 21. Abnormal emergence of Eumaeus atala Poey adult. Eclosion was prevented by silk spun by later pupating larvae. Photograph by Sandy Koi, University of Florida.
Figure 22. Abnormal Eumaeus atala Poey adult with incompletely expanded wings. Photograph by Sandy Koi, University of Florida.
Fire
Fire (both naturally occurring and prescribed) may impact populations of both Atala butterflies and their coontie hosts. Negrón-Ortiz & Gorchov (1996) studied the effects of controlled burns on the survival of coonties and the echo moth, Seirarctia echo JE Smith, a cycad herbivore, but the effects of fire on Atala populations in the pine rocklands of South Florida have not yet been studied. Schwartz (1888) mentioned that “untold thousands of [Atalas] are destroyed by the fires which frequently sweep through the pinewoods.” The management plan for Miami-Dade County’s pine rocklands, an Environmentally Endangered Land, reports that the accepted historical frequency of naturally occurring fires is once every 3-7 years (Miami-Dade 2007). Fires greatly impact the animals, insects, and plants of this ecosystem, but there has been little research on these effects on the native biota, except the vegetation. It is known that less frequent fires provide more fuel, and hence greater intensity (Miami-Dade 2007). A recent paper provides important information on fire intensity, general pupal survival and fire management for lepidopteran survival (Thom 2015).
Edwards (2001) discussed the effect of “time-since-burn” on plant communities and butterflies in pine flatwoods, finding proper burn management protocols can increase populations of fire-dependent plants and native butterfly species. Recovery time for the plants post-fire directly affected the butterfly species that use them as nectar or host plants. One species, the dusted skipper, Atrytonopsis hianna loammii Scudder, showed an increase in populations 1-3 years post-burn in spite of a decrease immediately post-fire due to nectar and host plant scarcity and direct mortality. Edwards (2001) advised that proper burn rotation around areas housing butterfly species provide refugia for the populaton’s recovery. If a protected colony is nearby, butterflies will quickly re-colonize the burned area once the plants have recovered.
Management (Back to Top)
Careful cultural and physical Integrated Pest Management (IPM) strategies help prevent excessive damage to valuable or ornamental Zamia plants, while maintaining and stabilizing the Atala population at the same time.
Coontie plants are usually hardy and pest-resistant, but may be susceptible to scales, sooty mold and mealy bugs when under stress, which can be aggravated by considerable herbivory damage caused by Atala caterpillars. Knowledge of the expected survival and mortality rates of the Atala butterfly may help determine management decisions.
Recent studies (Koi 2013) indicate that the average hatch rate of Atala butterfly eggs is about 36% and the average survival rate of hatched larvae is approximately 58%. Once pupae are formed, however, 94% are expected to successfully emerge as adults.
Reducing egg numbers will reduce larval herbivory damage. Hand-removal of Atala eggs may be required to reduce an expected population explosion. Because the eggs do not adhere strongly to the waxy surface of coontie leaves, they are easily dislodged manually. The eggs can be frozen or simply left in the substrate where ants and other animals will likely eat them. Although it can be time-consuming, hand-removal is certainly less expensive than buying new cycads. If new larvae hatch in the ground, they will most likely be unable to find food before dying as they must find food within a few hours after eclosure to survive.
Pesticides to control unwanted insect pests such as mealy bugs should be used sparingly, to protect the butterfly adults and larvae, and the coontie pollinators. Treatments with the bacterium Bacillus thuringiensis, which is effective against the larvae of many Lepidoptera larvae, does not appear to be effective against Atala larvae (Culbert 1994).
Acknowledgements (Back to Top)
The authors thank James Maruniak (University of Florida) for verifying the NPV pathogen in Atala larvae and Drion Boucias (University of Florida) for identifying fungal bodies in Atala eggs.
Selected References (Back to Top)
- Atlas of Florida Vascular Plants. 2015. Institute for Systematic Botany. University of South Florida. Tampa, Florida.
- Baggett HD. 1982. Florida atala. In: Franz R, ed., Rare and Endangered Biota of Florida. Vol. 6. Invertebrates. University Presses of Florida. Gainesville, Florida. pp. 75-77.
- Bowers MD, Larin Z. 1989. Acquired chemical defense in the lycaenid butterfly Eumaeus atala. Journal of Chemical Ecology 15: 1133-1146.
- Bowers MD, Farley S. 1990. The behaviour of grey jays, Perisoreus canadensis, towards palatable and unpalatable Lepidoptera. Animal Behaviour 39: 699-705.
- Coile NC. 2000. Notes on Florida’s endangered and threatened plants. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Bureau of Entomology, Nematology and Plant Pathology-Botany Section. Contribution No. 38, 2nd edition. 95 p.
- Coile NC, Garland MA. 2003. Notes on Florida’s endangered and threatened plants. Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Bureau of Entomology, Nematology and Plant Pathology-Botany Section. Contribution No. 38, 4th edition. 122 p.
- Culbert DF. 1994. An IPM approach for the control of atala (Eumaeus atala) on Florida coonties (Zamia floridana). Proceedings of the Florida State Horticultural Society 107: 427-430.
- Culbert DF. (1995). Florida coonties and atala butterflies. University of Florida EDIS. (12 February 2019)
- Dehgan B. 2002. More cycads suited for the south. Ornamental Outlook, May, pp. 12-18.
- Doucet SM, Meadows MG. 2009. Iridescence: A functional perspective. Journal of the Royal Society Interface (Interface Focus Supplement) 6: S115-S132. (18 July 2015)
- Edwards AM. 2001. The effect of time-since-burn on butterflies in a pine flatwoods. Masters’ thesis. Florida Atlantic University. Aug. 56 p.
- Flora of North America. eFloras.org. http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=233501359 (18 July 2015)
- Hammer RL. 1996. New food plants for Eumaeus atala in Florida. News of the Lepidopterists' Society 38: 10. (18 July 2015)
- Haynes JL. 2000. Cycads in the south ‘Florida landscape’. Proceedings of the Florida State Horticultural Society 113: 309-311.
- Hinton HE. 1948. Sound production in the lepidopterous pupae. Entomologist 81: 254-269.
- Hooper PT. 1978. Cycad poisoning in Australia—etiology and pathology. In: RF Keeler, KR van Kampen and LF James, eds. Effects of poisonous plants on livestock. Academic Press, New York. pp. 337-347.
- Hoegh-Guldberg O. 1972. Pupal sound production of some Lycaenidae. Structure and sound. Journal of Research on the Lepidoptera 10: 127-147.
- Klots AB. 1951. A Field Guide to the Butterflies of North America, East of the Great Plains. Houghton Mifflin, Boston.
- Koi S, Daniels J. 2015. New and revised life history of the Florida hairstreak Eumaeus atala (Lepidoptera: Lycaenidae) with notes on its current conservation status. Florida Entomologist 98: 1134-1147.
- Koi SE. 2013. Ecology and conservation of Eumaeus atala Poey 1832 (Lepidoptera: Lycaenidae). Master’s thesis. University of Florida. 295 pp.
- Koi S. 2008. Nectar sources for E. atala. Florida Entomologist 91: 118-120.
- Landolt PJ. 1984. The Florida atala butterfly, Eumaeus atala florida Rueber (Lepidoptera: Lycaenidae), in Dade County, Florida. Florida Entomologist 67: 570-571.
- Laudonnière R. 1975. Three Voyages. Translated by Charles E. Bennet. University of Florida Press, Gainesville.
- Miami-Dade County Environmentally Endangered Lands Program Management Plan. 2007. Part II: Management of specific habitat types. Chapter 1: The pine rockland habitat. 70 pp.
- Minno MC, Emmel TC. 1993. Butterflies of the Florida Keys. Scientific Publishers, Gainesville, Florida.
- Minno MC, Butler JF, Hall DW. 2005. Florida butterfly caterpillars and their host plants. University Press of Florida. Gainesville, Florida. 360 pp.
- Nash RJ, Bell EA, Ackery PR. 1992. The protective role of cycasin in cycad-feeding lepidoptera. Phytochemistry 31: 1955-1957.
- Negrón-Ortiz V, Gorchov DL. 1996. Effect of fire season on Zamia pumila L. in pinelands of Everglades National Park, FL: preliminary findings. American Journal of Botany 83: 85. Abstract #245.
- Opler PA, Krizek GO. 1984. Butterflies East of the Great Plains: An Illustrated Natural History. The Johns Hopkins University Press. Baltimore, MD. 294 pp.
- Plants Database. 2019. USDA Natural Resources Conservation Service. (12 February 2019)
- Ramírez-Restrepo, L, Koi S, MacGregor-Fors I. 2015. Tales of conservation in cities: The case of Eumaeus butterflies and their threatened cycad hostplants. Conservation Biology. In review.
- Rawson G. 1961. The recent rediscovery of Eumaeus atala (Lycaenidae) in Florida. Journal of the Lepidopterists' Society 15: 237-244.
- Richardson ML, Mitchell RF, Reagel PF, Hanks LM. 2010. Causes and consequences of cannibalism in noncarnivorous insects. Annual Review of Entomology 55:39-53.
- Rothschild M. 1992. Egg protection by the atala hairstreak butterfly (Eumaeus atala florida). Phytochemistry 31: 1959-1960.
- Rothschild M, Nash RJ, Bell EA. 1986. Cycasin in the endangered butterfly Eumaeus atala florida. Phytochemistry 25: 1853-1854.
- Schneider D. 2001. Cycads-Palmferns-and their insects (Herbivores and Pollinators). European Symposium for Insect Taste and Olfaction. (no longer online)
- Schneider D, Wink M, Sporer F, Lounibos P. 2002. Cycads: Their evolution, toxins, herbivores and insect pollinators. Naturwissenschaften 89: 281-294.
- Schwartz EA. 1888. Notes on Eumaeus atala. Insect Life. 1: 37-40.
- Scott JA. 1986. The Butterflies of North America: A Natural History and Field Guide. Stanford University Press. Stanford, California. 583 pp.
- Smith DS, Miller LD, Miller JY. 1994. Butterflies of the West Indies and South Florida. University of Oxford Press. 346 pp.
- Smith EM. 2002. The effect of season, host plant protection and ant predators on the survival of Eumaeus atala (Lycaenidae) in re-establishments. Journal of the Lepidopterists Society 56: 272-276.
- Thom MD, Daniels JC, Kobziar LN, Colburn JR. 2015. Can butterflies evade fire? Pupa location and heat tolerance in fire prone habitats of Forida. PlosOne. DOI: 10.1371/journal.pone.0126755.
- Ward DB. 2015. What is the REAL Zamia pumila? The Palmetto 32: 14-15.
- Wilts BC, Leerouwer HL, Stavenga DG. 2009. Imaging scatterometry and microspectrophotometry of lycaenid butterfly wing scales with perforated multilayers. Journal of the Royal Society Interface (Interface Focus Supplement) 6: S185-S192. (12 February 2019)
- Yagi F. 2004. Azoxyglycoside content and β-glycosidase activities in leaves of various cycads. Phytochemistry 65: 3243-3247.