common name: lively cuckoo bee (suggested common name)
scientific name: Nomada fervida Smith (Insecta: Hymenoptera: Apidae)
Introduction - Synonymy - Distribution - Description and Life Cycle - Economic Importance - Selected References
Introduction (Back to Top)
“Cuckoo bee” is a common name ascribed to many different kleptoparasitic bee species. Latin for “parasitism by theft”, kleptoparasitic bees steal resources, usually pollen, that have been collected by a host bee. Of the over 3,700 parasitic bee species that have been described (Cane 1983), at least 850 of them belong to the largest kleptoparasitic genus, Nomada. Nomada species are host-specific and often parasitize one to several other closely-related species (Michener 2000). This is consistent with “Emery’s rule” (Emery 1909), which states that social parasites tend to be a sister species to, or at the very least a close relative of, their host. While the host of Nomada fervida is unknown, its close relatives parasitize metallic green bees (Agapostemon spp.). Like Nomada fervida, Agapostemon splendens is restricted to sandy habitats and is thought to be the primary host of this kleptoparasitic bee species (Droege et al. 2010).
Figure 1. Profile view of a female Nomada fervida Smith collected along the Atlantic coast at Fort Matanzas National Monument, Florida. Photograph by Amber Reese from the USGS Bee Inventory and Monitoring Lab.
Figure 2. Frontal view of a female Nomada fervida Smith collected along the Atlantic coast at Fort Matanzas National Monument, Florida. Photograph by Amber Reese from the USGS Bee Inventory and Monitoring Lab.
Figure 3. Dorsal view of a female Nomada fervida Smith collected along the Atlantic coast at Fort Matanzas National Monument, Florida. Photograph by Amber Reese from the USGS Bee Inventory and Monitoring Lab.
Synonymy (Back to Top)
Nomada crassula Cockerell, 1903
Nomada wisconsinensis Graenicher, 1911
Hypochrotaenia (Micronomada) fervida (Smith 1854)
Hypochrotaenia (Micronomada) crassula (Cockerell 1903)
Hypochrotaenia (Micronomada) wisconsinensis (Graenicher 1911)
Distribution (Back to Top)
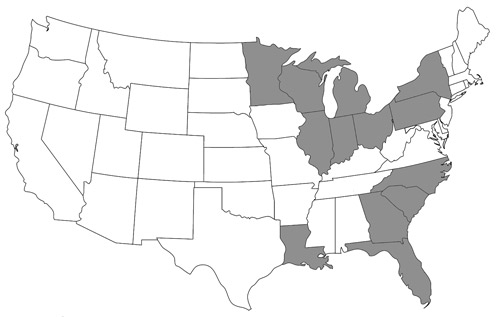
Figure 4. State distribution map of Nomada fervida Smith in the United States constructed using data from Droege et al. 2010 and specimen records in Table 1. States colored grey are indicative of known species records.
Nomada fervida is a relatively uncommon sand specialist species. Droege et al. (2010) reported two known populations in North America: (1) in Florida and Georgia and (2) around the Great Lakes, in Wisconsin, Michigan, Illinois, Indiana, and Ontario. Additional specimens were later identified from South Carolina, North Carolina, Louisiana, Ohio, New York, Pennsylvania, and Minnesota (Figure 4). This species might also be present in similar sandy habitats including those in between the northern and southern populations, the Nebraska Sandhills, New Jersey Pine Barrens, Long Island, Cape Cod, and the dunes of the western Great Plains (Droege et al. 2010).
Table 1. Locality data of Nomada fervida Smith specimens from the following collections/databases: American Museum of Natural History (AMNH); Bee Biology and Systematics Laboratory (BBSL); Global Biodiversity Information Facility (GBIF); Snow Entomology Collection, University of Kansas (KSEM); MaLisa Spring (MLSC); and Rutgers University Arthropod Collection (RUAC).
Identification # Locality AMNH_BEE00114626 FL, Miami-Dade Co.; R. Hardy Matheson Preserve BBSL520835 FL, Highlands Co.; Archbold Biological Station RUAC_ENT00000177 FL, Highlands Co.; Highlands Hammock S. P. AMNH_BEES24000 FL, Polk Co.; Lake Haines BBSL520834 FL, Orange Co.; Orlando BBSL520832 FL, Lake Co.; Leesburg, UF Experimental Station AMNH_BEE00022292 FL, Lake Co.; Leesburg, UF Experimental Station USGS_DRO344977 FL, St. Johns Co.; Fort Matanzas National Monument AMNH_BEE00062952 FL, Alachua Co.; Archer, S of Kanapaha Prairie AMNH_BEE00062953 FL, Alachua Co.; Gainesville, Lake Alice BBSL520833 FL, Alachua Co.; Gainesville, UF campus BBSL520830 GA, Charlton Co.; Okefenokee Swamp AMNH_BEES23998 SC, Orangeburg Co. KSEM507912 NC, Swain Co.; Smokemont AMNH_BEES24002 LA, West Feliciana Co. AMNH_BEES23997 NY, Madison Co. GBIF436543288 PA, Erie Co.; Presque Isle MLSC604 OH, Washington Co.; Beiser Field Station GBIF311368815 OH, Ottawa Co.; Catawba Island AMNH_BEES23994 MI, Midland Co. AMNH_BEES23996 IN, Marion Co. GBIF436543589 IL, Will Co.; 1mi. E of Braidwood AMNH_BEES23995 IL, Sangamon Co. GBIF436543593 IL, Mason Co.; Sand Ridge S. F. GBIF436543287 IL, Mason Co.; Sand Ridge S. F. BBSL521057 IL, Mason Co.; Sand Ridge S. F. AMNH_BEES23993 WI, Marathon Co. AMNH_BEES24001 MN, Aitkin Co.
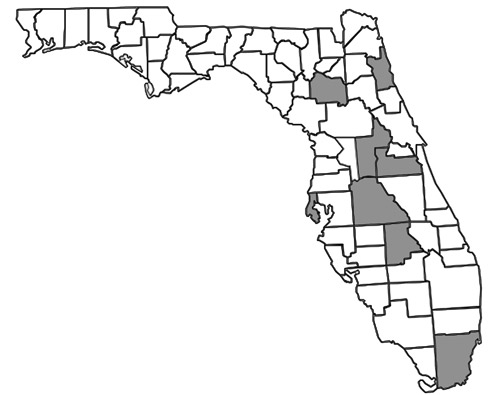
In Florida (Figure 5), most recorded specimens are from inland habitats with the exception of three specimens collected from coastal dunes: (1) in St. Petersburg (Droege et al. 2010), (2) at the R. Hardy Matheson Preserve in Miami-Dade County, and (3) at Fort Matanzas National Monument. Surveys have shown greater abundance and diversity of Nomada species in the northern parts of Florida than in south Florida (Hall & Ascher 2010). As with any obligate kleptoparasitic species, it is important to remember that the distribution of Nomada fervida is limited to the distribution of its host species.
Figure 5. County distribution map of Nomada fervida Smith in Florida constructed using data from Droege et al. (2010) and specimen records from Table 1.
Description (Back to Top)
Most kleptoparasitic bee species have poorly developed pollen-gathering structures or have lost them entirely. Nomadine bees, including Nomada fervida, are no exception, having lost the branched hairs and scopa (pollen carrying apparatus) characteristic of most bees, thereby becoming more wasp-like in appearance (Michener 2000). Nomada species have a long, slender body with yellow-to-orange markings. Two characters useful for separating Nomada fervida from other Nomada species include the following: (1) the scutellum (dorsal surface of the second mesosomal segment) is yellow-to-orange, and (2) the propodeum (last mesosomal segment) and propodeal triangle are entirely black (Figure 6).
Figure 6. Dorsal view of the scutellum and propodeum of a female Nomada fervida Smith. Photograph by Molly Rightmyer.
Spines and spine-like setae (hairs) on the legs are also useful for distinguishing Nomada species (Droege et al. 2010). Nomada fervida and other Nomada species in the eastern United States have a distinct spine on the front coxa that points posteriorly. Females (Figure 7) of Nomada fervida have a dense patch of over 20 reddish, spine-like setae on the outer apical margin of their hind tibia (Figure 8). Males (Figure 9) also have these setae (Figure 10), but this character is much more noticeable in females. Females also can be distinguished from males by the presence of a sting (modified ovipositor) at the posterior end of the abdomen, whereas males, instead, have distinct external genitalia.
Figure 7. Dorsal view of a female Nomada fervida Smith. Photograph by Molly Rightmyer.
Figure 8. Spine-like setae on the outer apical margin of the hind tibia of a female Nomada fervida Smith. Photograph by Molly Rightmyer.
Figure 9. Dorsal view of a male Nomada fervida Smith. Photograph by Molly Rightmyer.

Figure 10. Spine-like setae on the outer apical margin of the hind tibia of a female Nomada fervida Smith. Photograph by Molly Rightmyer.
Description of the intraspecific morphological variation observed across the geographic distribution of Nomada fervida is provided by Droege et al. 2010. Individuals of Nomada fervida from the southern population more frequently have a burnt orange tinge in their yellow bands and spots, while this tinge is less apparent in those from the Great Lakes population. The yellow-to-orange markings in individuals from the southern population are also typically much less pronounced and partially reduced on the head, ventral surface of the thorax (mesepisternum), and abdomen than in individuals from the northern population. However, all color variations can be seen within both populations. No significant variation exists in cuticular sculpturing, body size, proportions of flagellar (antennal) segments, or the number of spine-like setae on the hind tibia. The northern population was thought to be a separate species (Nomada wisconsinensis) until 2010. DNA barcoding and overlap in morphological variation across the two populations provided support that the northern population is also Nomada fervida.
Biology (Back to Top)
The host species of kleptoparasitic bees range from solitary to primitively eusocial (Cane 1983). Kleptoparasitism in bees has evolved independently over 16 times in species that target social hosts and over 31 times in species with solitary hosts (Michener 2000). Communal nesting behavior in the host likely increases the difficulty for Nomada species to infiltrate the nest, since several adults are involved with brood care in communal species. Some metallic green bees (Agapostemon spp.) are communal, but the primary host of Nomada fervida is believed to be the solitary species Agapostemon splendens (Eickwort 1981).
Males of Nomada species often have cephalic secretions that are very similar to the volatiles released from the associated host female’s Dufour’s gland (Tengö & Bergström 1977). Secretions from the Dufour’s gland are used by the host to line brood cells and are detected by the female cuckoo bee when she searches for a host burrow to provision her eggs. It appears as though the male cephalic secretions functionally mimic the host volatiles. Interestingly, the secretions of female cuckoo bees are very different from those of male conspecifics. Two proposed hypotheses (Michener 2000) to the nature of this phenomenon include: (1) the female cuckoo bee learns the host’s odor from the male when she mates with him, and (2) the female acquires the odor from the male during copulation, which allows her to infiltrate the host nest undetected. Alternatively, the female cuckoo bee may already be capable of detecting host odors and the male’s secretions increase his chances of attracting a female with whom to copulate, thereby increasing his reproductive output.
Adult cuckoo bees are known to visit flowers to feed upon nectar. Some specimens from Table 1 are associated with flowers from the following genera: Bidens, Ceanothus, Melilotus, and Polygonum. Other records of associated flowers include: Balduina atropurpurea (Adams et al. 2010), Balduina augustifolia and Pityopsis graminifolia (Deyrup et al. 2002), and Symphyotrichum oblongifolium (Figure 11).
Figure 11. A male Nomada fervida Smith feeding on nectar from 'Raydon’s Favorite' aromatic aster (Symphyotrichum oblongifolium). Photograph by Fitz Clarke.
Life Cycle (Back to Top)
Like all other Hymenoptera, cuckoo bees are holometabolous and undergo complete metamorphosis, with the following life stages (in order): egg, larva, pupa, and adult. Mated female cuckoo bees hover low over the ground and use olfactory cues to locate suitable host burrows (Cane 1983). Once she has found a burrow, she waits for the adult bee to leave before invading the nest to oviposit her own eggs. One-to-two eggs are typically deposited into the wall of a single egg cell. Cuckoo bee first-instar larvae are prognathous (have forward-facing jaws) and have long, sickle-shaped mandibles. Once the cuckoo bee egg hatches, the larva will use its specialized mandibles to kill other eggs or larvae—including conspecifics—and then feed upon the pollen ball (Michener 2000). The larva will then pupate within the host cell and later emerge as an adult.
Economic Importance (Back to Top)
Since adults visit flowers for nectar, it is possible that cuckoo bees have some involvement in pollination. However, their distinct lack of pollen-gathering structures and behavior probably renders them relatively poor pollinators. Hosts of Nomada species gather pollen and therefore are likely pollinators. However, kleptoparasites are a natural part of the ecosystem and there is no evidence that cuckoo bees are a major threat to pollinator populations. Focus on the preservation of pollinator populations should instead be directed at mitigating damage from more impactful drivers, including habitat loss and fragmentation, climate change, invasive species, pathogens, and pesticides (Potts et al. 2010).
Selected References (Back to Top)
- Adams LD, Buchmann S, Howell AD, Tsang, J. 2010. A study of insect pollinators associated with DoD TER-S flowering plants, including identification of habitat types where they co-occur by military installation in the southeastern United States. Department of Defense Legacy Resource Management Program. Project # 09-391.
- Cane JH. 1983. Olfactory evaluation of Andrena host nest suitability by kleptoparasitic Nomada bees (Hymenoptera: Apoidea). Animal Behavior 31: 138-144.
- Deyrup M, Edirisinghe J, Norden B. 2002. The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea). Insecta Mundi 16: 87-120.
- Droege S, Rightmyer MG, Sheffield CS, Brady SG. 2010. New synonymies in the bee genus Nomada from North America. Zootaxa 2661: 1-32.
- Eickwort GC. 1981. Aspects of the nesting biology of five Nearctic species of Agapostemon (Hymenoptera: Halictidae). Journal of the Kansas Entomological Society. 54: 337-351.
- Emery C. 1909. Über den Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen. Biologisches Centralblatt 29: 352-362.
- Hall HG, Ascher JS. 2010. Survey of bees (Hymenoptera: Apoidea: Anthophila) in natural areas of Alachua County in north-central Florida. Florida Entomologist 93: 609-629.
- Michener CD. 2000. The Bees of the World: Second Edition. Baltimore: The Johns Hopkins University Press.
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: Trends, impacts and drivers. Trends in Ecology and Evolution 25: 345-353.
- Tengö J, Bergström G. 1977. Cleptoparasitism and odor mimetism in bees: Do Nomada males imitate odor of Andrena females? Science 196: 1117-1119.