common name: pipevine swallowtail, blue swallowtail
scientific name: Battus philenor (Linnaeus 1771) (Insecta: Lepidoptera: Papilionidae: Papilioninae: Troidini)
Introduction - Nomenclature - Distribution - Description - Host Plants - Life Cycle - Natural Enemies - Defenses - Mimicry - Acknowledgements - Selected References
Introduction (Back to Top)
The pipevine swallowtail, Battus philenor (L.), is one of our most beautiful swallowtails. It is also known as the blue swallowtail (e.g., Howe 1988, Iftner et al. 1992). Its life cycle was beautifully illustrated during the 18th century by John Abbot (Smith 1797) (Figure 1).
Figure 1. Life cycle of the pipevine swallowtail, Battus philenor (L.). Drawing by John Abbot (From Smith 1797). (For aesthetic reasons, the color temperature of the image was adjusted to compensate for yellowing of the manuscript due to age).
Nomenclature (Back to Top)
The pipevine swallowtail was originally described by Linnaeus (1771) and placed in the genus Papilio with the other swallowtails. It was later moved to the genus Battus (Scopoli 1777). The name “Battus” is from Battus I, founder of the ancient Greek colony Cyrenaica and its capital, Cyrene, in Africa. The specific epithet is from the Greek word “philenor” which means fond of husband or conjugal (Opler & Krizek 1984).
Larvae of the pipevine swallowtail and those of the other swallowtails belonging to the tribe Troidini feed on plants in the genus Aristolochia and are commonly referred to as the Aristolochia swallowtails.
For synonymy, see the excerpt from Pelham (2008) at the Butterflies of America web page on Battus philenor (Accessed December 23, 2016).
Distribution (Back to Top)
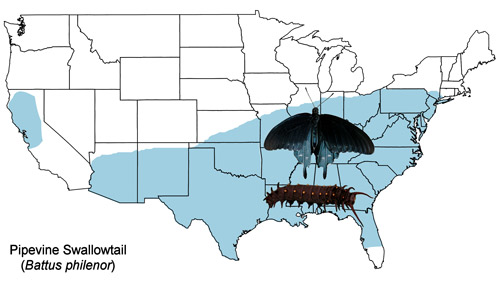
The U. S. distribution of the pipevine swallowtail extends from southern Connecticut south to central Florida and west to Arizona with an isolated population in northern California (Figure 2). The pipevine swallowtail is also found northward to southeastern Ontario, Canada and southward to southern Mexico (Cech & Tudor 2005, Opler & Malikul 1998, Scott 1986).
Figure 2. Pipevine swallowtail, Battus philenor (L.), U.S. distribution map.
Published distribution maps (e.g., Brock & Kaufman 2003, Scott 1986, Moth Photographers Group, Battus philenor species page, Butterflies and Moths of North America) differ due to records of adult “strays” and to planting of larval host plants as ornamentals (Iftner et al. 1992, Layberry et al. 1998) outside their native ranges. For example, the only documented records for Battus philenor larvae in Ontario are from Aristolochia macrophylla vines planted as ornamentals (Layberry et al. 1998).
Description (Back to Top)
Adults: The wingspread is 2 3/4-5 1/8 in. (72-132 mm) (Opler & Malikul 1992). The dorsal surfaces of the wings of males are mostly black with blue or blue-green iridescence on the hind wings (Figure 3). The dorsal aspect of the hindwings of females (Figure 4) has duller iridescence than that of males. There are light marginal and sub-marginal spots on both forewings and hindwings. The ventral hind wings of both sexes have blue iridescence and a row of seven bright orange sub-marginal spots (Figures 4 & 5). There is a row of white spots on the lateral aspect of the abdomen (Figure 5).
Figure 3. Adult male pipevine swallowtail, Battus philenor (L.), dorsal view showing brilliant iridescence. Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 4. Adult female pipevine swallowtail, Battus philenor (L.), dorsal (left) and ventral (right) views. Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 5. Newly-emerged adult male pipevine swallowtail, Battus philenor (L.), with wings folded showing undersides of the wings and white spots on abdomen. Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
California specimens are smaller, with hairy bodies. Their larvae also have shorter thoracic filaments than those of eastern specimens. The California population also differs from eastern populations in some aspects of biology (Sims & Shapiro 1983b) and may represent a different subspecies, Battus philenor hirsuta (Sourakov & Daniels 2002). According to Field (1938, p. 200), individuals of the spring generation in Kansas resemble those from California populations.
Eggs: The eggs are reddish-orange (Figure 6) in contrast to those of the related Polydamas swallowtail, Battus polydamas (L.), which are yellow or yellowish-orange. The eggs of all Aristolochia (troidine) swallowtails are partially covered by a hard, nutritious secretion that is usually laid down in vertical bands (Comstock & Grimshawe 1935, Tyler et al. 1994). There are numerous relatively large droplets on the bands. The secretion is produced by a large gland that lies above the female’s ovipositor duct (Tyler et al. 1994).
Figure 6. Eggs of the pipevine swallowtail, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Larvae: Full-grown larvae are approximately 50 mm (about 2 in.) in length (Minno et al. 2005, Stehr 1987, Wagner 2005).
First instar larvae have numerous short orange tubercles – each with a single seta (Figure 7). Second instars have longer tubules - each bearing multiple setae (Figure 8). The tubercles of third and fourth instars are proportionately longer, and the exoskeleton takes on a slightly glossy appearance (Figure 9).
Figure 7. First instar larva of the pipevine swallowtail, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 8. Second instar larva of the pipevine swallowtail, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 9. Third instar larva of the pipevine swallowtail, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Full-grown larvae are typically dark brown to black with sub-dorsal and lateral rows of bright orange tubercles (Figure 10), but some are red (Figure 11). In western Texas and southern Arizona, the red form predominates under conditions of higher temperature (above about 30°C) (Nice & Fordyce 2006, Nielsen & Papaj 2015, Papaj & Newsome 2005). The factor(s) responsible for red larvae in the eastern U.S. has not been investigated. The red larva in Figure 11 was photographed in Gainesville, Florida on July 29, 2016, during the heat of the summer.
Lateral tubercles on the thorax and abdominal segments 2, 7, 8, and 9 of larvae are modified into elongated filaments. The filaments on the prothorax are particularly long (Scott. 1986, Minno et al. 2005, Stehr 1987). Full-grown larvae are covered with fine hairs that give them a velvety appearance (Howe 1975).
Figure 10. Full-grown dark larva of the pipevine swallowtail, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 11. Full-grown red larva of the pipevine swallowtail, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
The sex of larvae of all ages can be determined by the structure of sex-specific pits on the ventral surface of the 8th and 9th abdominal segments (Underwood 1994).
Pupae: Pupae may be either green or brown (Figure 12). Unlike pupae of other swallowtails, the sides of the bodies of Battus pupae are widened into lateral flanges. In the lateral views of pupae shown in Figure 12, the lateral flanges appear as bluish-purple ridges along the anterior half of the abdomen.
Figure 12. Green pupa and brown pupa of the pipevine swallowtail, Battus philenor (L.). Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Host Plants (Back to Top)
Larval host plants: Pipevine swallowtail larvae feed on plants belonging to the genus Aristolochia in the family Aristolochiaceae (Cech & Tudor 2005, Minno et al. 2005, Opler & Krizek 1984, Scott 1986). Reports in the literature (e.g., Arnett 2000, Howe 1975, Robinson et al. undated, Schull 1987, Tyler 1975) of larvae feeding on wild ginger, Asarum canadense L. (Aristolochiaceae), knotweed, Polygonum (Polygonaceae), and morning glories, Ipomoea (Convolvulaceae), are probably erroneous (Heitzman & Heitzman 1987, Opler & Krizek 1984, Scott 1986) and likely due to misidentification of the host plant by Blatchley (1896) in the case of Asarum (see Weintraub 1995) and to wandering larvae for the other plant species.
Aristolochia species are commonly known as pipevines or Dutchman’s pipes because the flowers of some species are shaped like tobacco pipes (Figure 13). They are also known as birthworts (“wort” is Old English for herbaceous plant) because of their historical use in child birth. The name Aristolochia is derived from the Greek roots aristos (best) and lochia (delivery or child birth) (Crosswhite & Crosswhite 1985, Flora of North America undated). All Aristolochiaceae are believed to contain pharmacologically active aristolochic acids (Chen & Zhu 1987).
Figure 13. Unopened flower of woolly pipevine, Aristolochia tomentosa Sims, and tobacco pipe graphic showing the similarity of shapes. Photograph of flower bud by Donald W. Hall, Entomology and Nematology Department, University of Florida. Tobacco pipe graphic from PNG Clip Art (graphic modified and pasted into photograph by Donald W. Hall, University of Florida).
Although they are now officially banned in many countries, Aristolochia-derived herbal products or parts of the plants themselves are still used in many areas of the world for various conditions including snake bite, gastrointestinal problems, respiratory problems, wounds, infectious diseases, and fever (Austin 2004, Chen & Zhu 1987, Duke 2001, Schaneberg et al. 2002).
Virginia snakeroot, Aristolochia serpentaria L., has been used for many medical applications (Austin 2004, Duke 2001, Heinrich et al. 2009, Moerman 1998), and preparations made from it are still for sale online. An extract of the southwestern pipevine, Aristolochia watsonii Wooton & Standl., was the main ingredient in the snakeroot oil sold by traveling “snakeroot doctors” at medicine shows in the Old West during the 19th century (Crosswhite & Crosswhite 1985). Aristolochic acids in the products have been implicated as causative agents of renal toxicity and also may be carcinogenic (Heinrich et al. 2009, Schaneberg et al. 2002).
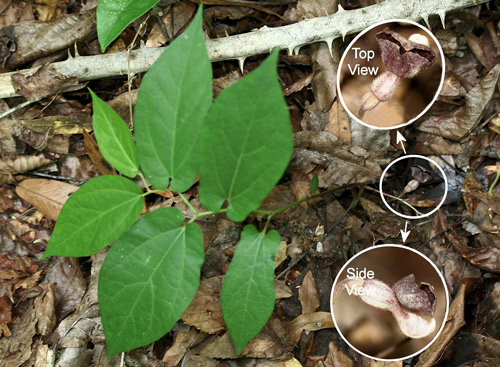
Aristolochia serpentaria L., ranges from central Florida northward in much of the eastern United States. In the Southeast, it has broad-leaved and narrow-leaved forms (Figures 14, 15 & 16). The broad-leaved form is more common in richer mesic forests while the narrow-leaved form is more common in drier, sandy areas (Allard 2002, D.W. Hall personal observation).
Figure 14. Virginia snakeroot, Aristolochia serpentaria L. (broad-leaved form), a host of the pipevine swallowtail caterpillar, Battus philenor (L.), with flower. Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
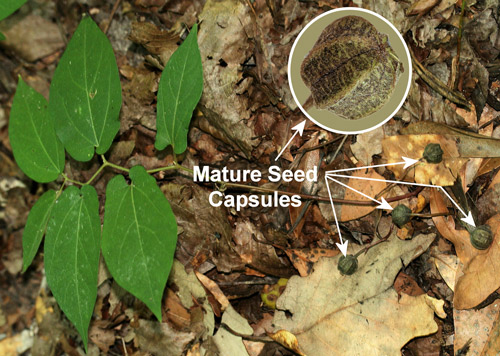
Figure 15. Virginia snakeroot, Aristolochia serpentaria L. (broad-leaved form), a host of the pipevine swallowtail caterpillar, Battus philenor (L.), with seed capsules. Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 16. Virginia snakeroot, Aristolochia serpentaria L. (narrow-leaved form), a host of the pipevine swallowtail caterpillar, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Aristolochia serpentaria L. and Aristolochia macrophylla Lam. (Figure 17) have been assayed for aristolochic acid, and both species were determined to contain significant quantities of aristolochic acid I (Schaneberg et al. 2002). Because of their toxicity and distastefulness, the aristolochic acids play a major role in the biology of pipevine swallowtails.
All of our native species of Aristolochia within the range of the pipevine swallowtail are documented larval hosts (for distribution maps see their respective USDA Plants Database species pages – links below):
- Virginia snakeroot, Aristolochia serpentaria L. (Figures 14, 15 &16) USDA Plants Database species page.
- Pipevine, Aristolochia macrophylla Lam. (synonym, Aristolochia durior Hill) (Figure 17) USDA Plants Database species page.
- Woolly Dutchman’s pipe, Aristolochia tomentosa Sims. (Figure 18) USDA Plants Database species page.
- Texas Dutchman’s pipe, Aristolochia reticulata Jacq. USDA Plants Database species page.
- Watson's Dutchman’s pipe, Aristolochia watsonii Wooton & Standl. USDA Plants Database species page.
- California Dutchman’s pipe, Aristolochia californica Torr. USDA Plants Database species page.
Figure 17. Pipevine, Aristolochia macrophylla Lam. (synonym, Aristolochia durior Hill) a host of the pipevine swallowtail caterpillar, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 18. Woolly Dutchman’s pipe, Aristolochia tomentosa Sims., a host of the pipevine swallowtail caterpillar, Battus philenor (L.), leaf (left), unopened flower (middle), opened flower (right). Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Various exotic Aristolochia species are planted as ornamentals because of their unusual and sometimes beautiful flowers. Some of these may be too toxic (or too distasteful) for pipevine swallowtail larvae and may be “death traps” for the larvae. Elegant Dutchman’s pipe or calico flower, Aristolochia elegans Mast (synonym, Aristolochia littoralis Parodi) (Figure 19), is attractive to female pipevine swallowtails for oviposition, but larvae usually do not survive on it (Kendall 1964, Scott 1986, Tyler 1975). Kendall (1964) suggested that Aristolochia elegans is probably only distasteful and not toxic to Battus philenor larvae and that the larvae probably die due to starvation because of their refusal to eat it. Therefore, planting of Aristolochia elegans and other exotic pipevines is not recommended where Battus philenor (L.) occurs (central Florida and northward).
Figure 19. Elegant Dutchman's pipe or calico flower, Aristolochia elegans M.T. Mast [synonym: Aristolochia littoralis Parodi], a cultured exotic which is a death trap for the pipevine swallowtail caterpillar, Battus philenor (L.). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Seed Dispersal: Aristolochia tomentosa and Aristolochia macrophylla are tall vines (Pfeifer 1966) with flattened seeds. Their seeds are probably dispersed by wind and possibly also by animals and flowing water. Two Russian Aristolochia vines with similar seeds that grow in similar habitats have been shown to be primarily dispersed by wind and water and to a lesser extent by birds (Nechaev & Nakonechnaya 2009).
Aristolochia serpentaria is a small herb. Its seeds have an attached oil body (elaiosome), and the seeds are dispersed by ants (myrmecochory) that collect the seeds for the edible elaiosomes (D.W. Hall, personal observation, Osorio 2010) (Figure 20).
Figure 20. Myrmecochory (ant dispersal) of Virginia snake root, Aristolochia serpentaria L., seed by trap-jaw ant, Odontomachus brunneus (Patton): a) dehisced (opened) seed capsule showing seeds with attached oil bodies (elaiosomes). b) trap-jaw ant, Odontomachus brunneus (Patton), removing seed from capsule c) trap-jaw ant, Odontomachus brunneus (Patton), carrying seed by the elaiosome. Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Both Aristolochia serpentaria and Aristolochia reticulata are small herbs, and their flowers (and seeds) are usually formed near the bases of the plants, usually underground or at least under leaf litter. Rausher and Feeny (1980) suggested that this may be an adaptation to protect the flowers and seeds from predation by Battus philenor caterpillars. However, it may also be an adaptation for dispersal of the seeds by ants. The mechanism of dispersal of seeds for Aristolochia reticulata is unknown but likely also involves ants.
Pollination:
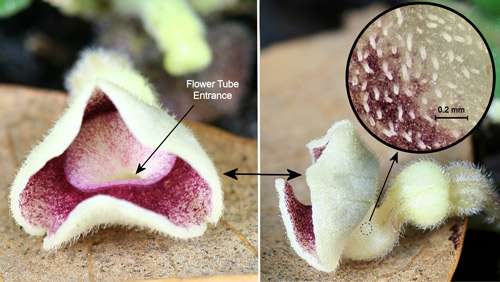
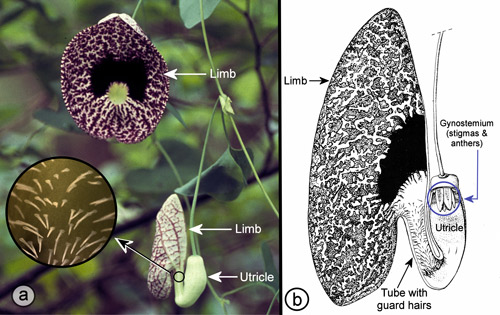
Insect pollination: Aristolochia species have fascinating pollination biologies (Proctor et al. 1996). When the flowers first open, the stigmas are receptive, but the anthers are not releasing pollen – a phenomenon known as protogyny (from the Greek roots "proto" [first] and "gyne" [female]). During the first day of bloom, the flowers are attractive to flies (usually small flies - often, but not always, scuttle flies in the family Phoridae [Hall & Brown 1993]) which enter the flowers carrying pollen from another flower. The flies are then prevented from leaving by the presence of downward projecting guard hairs or tiny spines and slippery surfaces in the tube of the flower (Proctor et al. 1996, Zomlefer 1994) (Figures 21, 22, & 23).
Figure 21. Virginia snakeroot, Aristolochia serpentaria L., flower: front view (left), side view (right), and inside of tube with slippery surface and small downward pointing hairs (inset). Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 22. Woolly pipevine, Aristolochia tomentosa Sims, flower (left) and longitudinal section (right) showing inside of tube with slippery surface and small downward pointing spines (inset). Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 23. Elegant pipevine, Aristolochia elegans M.T. Mast (synonym: Aristolochia littoralis Parodi). a ) flowers in front and side view orientations and downward pointing guard hairs (inset). b) drawing of longitudinal section of flower showing inside of tube with downward pointing guard hairs. Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida. Drawing by Margo Duncan.
On the second day (after pollination), the stigmas become non-receptive, and the anthers dehisce (open) dusting the flies with pollen. The guard hairs in the flower tubes wither, and in some species (e.g., Aristolochia gigantea Mart. & Zucc.) the flowers also droop into a more horizontal position (Burgess et al. 2004) releasing the flies that are now carrying the new pollen.
Cleistogamy: In addition to its open (chasmogamous) insect-pollinated flowers, Aristolochia sepentaria also has flowers that never open (cleistogamous) and are self-pollinated (Ahles 1959, Pfeifer 1966). The terms chasmogamous and cleistogamous are from Greek roots (“chasma” [open or gaping], “cleist”, [closed], and “gam”, [marriage]) (Borror 1960). Cleistogamy is actually a common phenomenon in angiosperms (flowering plants). It is known from at least 693 species in 228 genera and 50 families (Culley & Klooster 2007).
The cleistogamous flowers of Aristolochia serpentaria (Figures 16 & 24) and their resultant seed capsules (Figure 24) are little more than half the size of the chasmogamous flowers and their seed capsules (Figures 14 & 15). Also, the cleistogamous flowers do not have the characteristic “tobacco pipe” shape as they do not trap insects for pollination. Both cleistogamous and chasmogamous flowers often occur on the same plant. In contrast to the chasmogamous flowers, the cleistogamous flowers are often formed later in the season in some species (Mondal 2016) and higher on the plant (Figure 24) where those of Aristolochia serpentaria are more susceptible to being eaten by Battus philenor larvae.
Figure 24. Virginia snakeroot, Aristolochia serpentaria L., plant with insets showing sequential stages of development of cleistogamous flower to mature seed capsule. Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Nectar host plants: There are many plants that are valuable as nectar sources for butterflies. Pink and purple flowers (e.g., phlox [Phlox species], ironweed [Vernonia species], and thistles [Cirsium species]) are particularly attractive to pipevine swallowtails (Scott 1986). Minno and Minno (1999) have extensive lists of both native and exotic nectar plants for butterflies. When possible, native plants should be planted as nectar sources rather than exotics that have the potential to be invasive.
Most states have native plant societies that are valuable sources of information on native plants, and many also hold native plant sales. For Florida and the Deep South, the Florida Wildflowers Growers Cooperative is an excellent source of information and also has wildflower seeds for purchase.
To maximize butterfly populations in yards, both caterpillar hosts and nectar plants for adults should be planted.
Life Cycle (Back to Top)
There are three or more generations in the Deep South (Gulf of Mexico area and peninsular Florida) and two generations northward (Cech and Tudor 2005, Glassberg et al. 2000, Howe 1975).
Males suck moisture from mud to obtain nutrients (particularly sodium) and attraction to the mud appears to be enhanced by the presence of other males (Otis et al. 2006). Males transfer some of the sodium to the female in the spermatophore as a nuptial gift during mating (Otis et al. 2006).
Mating: Males patrol host plants and flyways to locate females (Lederhouse 1995). Courtship flights are slow with the male hovering above the female (Cech and Tudor 2005). Battus males have androconia (scent scales) hidden in a fold of the inner margin on the upper surface of the hind wing (Field 1938) (Figure 25). The scales are fluted - possibly to increase surface area for release of pheromone (Miller 1987, Racheli & Oliverio 1993). When courting, Battus males are reported to “helicopter” around the females while fanning the androconial chemicals over them (Tyler et al. 1994, p. 53). However, West (1983) reported an observation of mating of Battus philenor without fanning of the wings. He also described a method for hand-pairing them in captivity.
Figure 25. Pipevine swallowtail, Battus philenor (L.), male showing iridescent scales (left inset) and androconia (right inset). Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida. Lyle J. Buss assisted with the photograph of androconia.
The brilliant iridescent blue on the dorsal surface of the hind wings of males is believed to serve as a sexual signal for recognition and possibly for assessment of male quality by females (Rutowski et al. 2010, Rutowski & Rajyaguru 2013). For a general review of the functions of iridescence in animals, see Doucet and Meadows (2009).
Oviposition: Females search for larval host plants based on leaf shape (Allard & Papaj 1996, Rausher 1978). Plants with new leaf buds are more attractive for oviposition (Papaj 1986b). If females detect either eggs or larvae on a plant they avoid ovipositing on it (Rausher 1979).
Females will also investigate non-host plants with leaves shaped like those of the local host plants. In Florida, they frequently visit young Smilax (Smilacaceae) vines that resemble Aristolochia serpentaria (Feeny 1991). Papaj (1986a) observed a female examine a Smilax laurifolia L. plant repeatedly before finally depositing an egg on it. However, when he transferred larvae to Smilax, they all died. Oviposition mistakes by Lepidoptera are not uncommon. In fact, long-tailed skippers (Urbanus proteus [L.]) will oviposit on Aristolochia tomentosa, but the larvae are only able to survive on it during the early instars (Figure 26) and die before reaching maturity (Marc Minno [personal communication], D.W. Hall [personal observation]).
Figure 26. Early instar long-tailed skipper (Urbanus proteus [L.]) leaf shelter on woolly pipevine, Aristolochia tomentosa Sims. Inset: leaf shelter opened to show larva. Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Aristolochic acids I and II, the inositols D-(+)-pinitol and sequoyitol, and a monogalactosyl diglyceride (all isolated from Aristolochia macrophylla) were demonstrated to serve as synergistic contact oviposition stimulants for Battus philenor (Sachdev-Gupta et al. 1993). None of the chemicals by themselves had significant activity.
Eggs are laid on young foliage or on stems at the bases of leaves - either singly or in small groups on small vines or in larger groups on large vines (Allen 1997. Minno et al. 2005). Young larvae are gregarious, but older larvae are solitary (Allen 1997, Scott 1986).
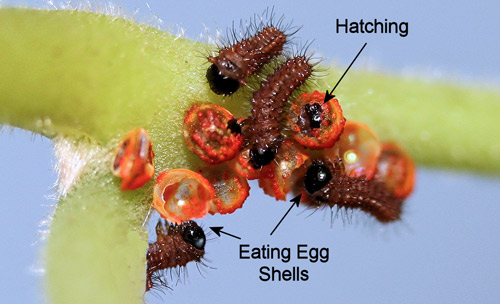
In Lepidoptera eggs, a small quantity of yolk remains trapped between two of the embryonic membranes (amniotic and serosa) (Barbier & Chauvin 1976) that remain inside the egg shells (chorions) after hatching. Soon after hatching, larvae eat the egg shells (Figure 27), and the residual yolk serves as their first meal (Richards & Davies 1977). Larvae also eat their exuviae after molting to conserve nutrients (Figure 28).
Figure 27. Pipevine swallowtail, Battus philenor (L.), newly-emerged first instar larvae eating egg shells (chorions). Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 28. Pipevine swallowtail, Battus philenor (L.), last instar larva eating its exuviae. Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Battus larvae commonly defoliate their host plants (Tyler et al. 1994) and need to wander in search of new plants. The elongated thoracic filaments of larvae have only tactile sensors and no chemosensory structures. These filaments and the lateral stemmata (eyes) appear to merely help larvae locate vertical objects, which must then be identified as host or non-host by the antennae and mouthparts (Kandori et al. 2015). Wandering larvae may find new host plants by detection of plant volatiles. Aristolochia volatiles have been shown to be attractive to larvae of Battus polydamas (Pinto et al. 2009).
When full-grown, larvae (prepupae) wander off the host plant to find a pupation site. Pupation occurs well off the ground - usually on tree trunks or on cliff faces in the Appalachians (West & Hazel 1979). Larvae rarely pupate on green substrates (Hazel 1995). Before pupation they spin a silk girdle for support and a silk pad which they grasp with their terminal prolegs (Figure 29). After splitting the old larval exoskeleton and wriggling free, the new pupa hooks the cremaster at the tip of its abdomen into the silk pad (Figure 29).
Figure 29. Pipevine swallowtail, Battus philenor (L.), prepupa beginning pupation. Note the silk girdle. Photograph by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Figure 30. Pipevine swallowtail, Battus philenor (L.), prepupa molting to pupal stage. Note the successive positions of the shed larval head capsule (arrows). Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Brown pupae predominate (Hazel & West 1979, West & Hazel 1979). Pupal color is partially influenced by the texture of the pupation substrate but not by the photoperiod during the larval stage (Hazel & West 1979). Short larval photoperiod induces pupal diapause (Hazel & West 1983).
Shortly before emergence of adults, the patterns of the wings and body show through the pupal exoskeleton (Figure 31a). The adult emerges through a series of cleavage lines: a mid-dorsal cleavage line on top of the thorax, lines between the head and thorax, and lines between the thorax and wings (Scott 1986) (Figure 31b-d). After emergence, the adult rests near the old pupal case (Figure 31e) while expanding the wings and joining the two halves of the proboscis (tongue) together. During this time, it also voids droplets of the red, liquid, pupal waste products (meconium) (Figure 31e [inset]).
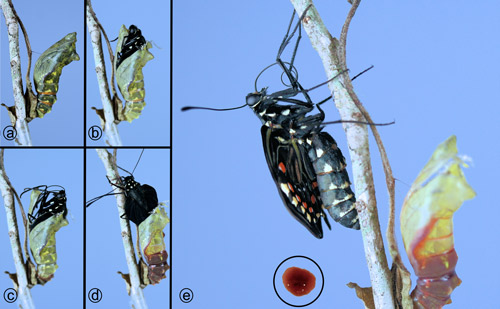
Figure 31. Pipevine swallowtail, Battus philenor L., stages in emergence of adult from pupal stage. Inset: droplet of pupal waste product (meconium). Photographs by Donald W. Hall, Entomology and Nematology Department, University of Florida.
Natural Enemies (Back to Top)
Stamp (1986) observed a third instar coccinellid larva (Hippodamia convergens Guerin-Meneville) feeding on a second instar pipevine swallowtail larva.
Records of parasitoids from Battus philenor are rare. However, there are records of at least one tachinid fly (Compsilura concinnata (Meigen)) (Arnaud 1978, p. 601) and two ichneumonid wasps (Theronia atalantae (Poda) and Apechthis annulicornis (Cresson)) (Sime 2000). Sims and Shapiro (1983a) reported the pupal parasitoid Brachymeria ovata (Say) (Hymenoptera: Chalcididae) from California Battus philenor and also noted significant mortality to pupae from a “disease” of unknown causes - possibly fungus.
Howe (1988) reported many species of birds eating Battus philenor larvae with “relish”. Anolis lizards will also eat the larvae (Odendaal et al. 1987).
Defenses (Back to Top)
Sequestered aristolochic acids: Battus philenor larvae sequester aristolochic acids from their host plants and the acids are passed on to the pupae, adults, and ultimately to eggs of the next generation (Dimarco et al. 2012, Dimarco & Fordyce 2013, Sime et al. 2000). The aristolochic acids are reported to render larvae, pupae, and adults unpalatable to some birds. Adults have tough bodies that allow them to survive being tasted (Scott 1986).
Contradictory reports on the palatability of larvae to birds may be due to differences in the ability of some populations (families) of Battus philenor to sequester aristolochic acids (Dimarco et al. 2012). Also, lower aristolochic acid concentrations in some Aristolochia species or variations in concentrations between individuals of the same species may explain the contradictions.
The chemical defenses appear to be effective against some parasitoids. The ichneumonid wasp Trogus pennator (Fabricius), which normally parasitizes papilionid larvae, rejected Battus philenor larvae after examining them with their antennae (Sime 2002).
Osmeteria: All United States swallowtail larvae have forked, eversible, horn-like organs behind the head known as osmeteria (singular, osmeterium). The osmeteria of Battus philenor larvae are bright yellow in all larval instars. Fifth instar Papilio species secrete isobutyric and 2-methyl butyric acids from their osmeteria (Ômura et al. 2006). The chemical makeup of the osmeterial secretion of fifth instar Battus philenor is unknown. However, the secretion of fourth and fifth instar larvae of the closely-related Battus polydamus reared on Aristolochia elegans is composed of the two sesquiterpenes: β-selinine and selin-11-en-4α-ol (Eisner et al. 1971). These compounds were not found in the host plant in detectable amounts and are assumed to be synthesized by the larvae instead of being sequestered (Eisner et al. 1971).
The osmeterial repellent of Papilio species is effective against ants (Eisner and Meinwald 1965), but Berenbaum et al. (1992) reported that soldier bugs (Hemiptera: Pentatomidae) could attack and eat swallowtail larvae (Papilio species) without evoking extrusion of the osmeteria.
Aposematism (warning coloration or appearance): The orange color on the ventral surface of the wings and the blue iridescence on both ventral and dorsal surfaces have been demonstrated to function as aposematic coloration against attack by vertebrate predators (Pegram et al. 2013, Pegram et al. 2015, Pegram & Rutowski 2014, Pegram & Rutowski 2016).
Mimicry (Back to Top)
Batesian mimicry: Batesian mimicry is a type of mimicry in which an edible species (the mimic) gains protection through its resemblance to a poisonous or distasteful species (the model). Battus philenor is believed to be the distasteful, aposematic model for the following edible swallowtail and brushfoot butterfly Batesian mimics (Brower 1958, Brower & Brower 1968, Poulton 1909):
Swallowtail butterfly mimics of Battus philenor:
Spicebush swallowtail (Pterourus [formerly Papilio] troilus) (Linnaeus)), (Papilionidae)
Eastern black swallowtail (Papilio polyxenes Fabricius), (Papilionidae)
Tiger swallowtail, (Pterourus [formerly Papilio] glaucus Linnaeus), (Papilionidae) – only the dark morph female is mimetic
Brushfoot butterfly mimics of Battus philenor:
Red-spotted purple (Limenitis arthemis astyanax (Drury)) (Nymphalidae)
Female spicebush and black swallowtails have blue on the dorsal surface of the hind wings and appear to be more convincing mimics than males when viewed dorsally. Male spicebush swallowtails have light green and those of black swallowtails have a lot of yellow and very little blue. However, males of these species have orange spots on the ventral surface of the hind wings and are probably mimetic when the wings are closed. Under experimental conditions, male and female black swallowtails were equally protected when their ventral sides were presented to captive blue jays, but males were eaten more frequently than females when their dorsal sides were presented (Codella and Lederhouse 1989).
Müllerian mimicry: Müllerian mimicry is a type of mimicry in which two or more species are similar in appearance and are mutually distasteful or dangerous. Therefore, each species acts simultaneously as a model and a mimic and gains protection from the resemblance. Scott (1986, p.73) suggested that Battus philenor larvae and certain millipedes may be Müllerian mimics. Some millipedes are dark colored like Battus philenor larvae and release hydrogen cyanide when threatened (Eisner 2003, Eisner et al. 2005).
Acknowledgements (Back to Top)
The author would like to acknowledge Howard Frank and Marc Minno for reviewing this article and offering helpful suggestions. Also, the assistance of Pam Drach and Laura Lamb, members of the southwestern chapter of the Indiana Native Plant Society, in providing foliage of woolly pipevine (Aristolochia tomentosa) for a Battus philenor caterpillar I was rearing during a trip to Indiana is greatly appreciated.
Selected References (Back to Top)
- Ahles H. 1959. Aristolochia serpentaria var. nashii as a new name for A. serpentaria var. hastata. Journal of the Elisha Mitchell Science Society 75: 130.
- Allard RA. 2002. Aristolochia serpentaria L. Virginia Snake Root. Conservation and Research Plan for New England. New England Wild Flower Society. Framingham, Massachusetts. 21 pp. (23 March 2020)
- Allard RA, Papaj DR. 1996. Learning of leaf shape by pipevine swallowtail butterflies: A test using artificial leaf models. Journal of Insect Behavior 9: 961-967.
- Allen TJ. 1997. The Butterflies of West Virginia and Their Caterpillars. University of Pittsburgh Press. Pittsburgh, Pennsylvania. 388 pp.
- Arnaud PH. 1978. A host-parasite catalog of North American Tachinidae (Diptera). United States Department of Agriculture Miscellaneous Publication 1319. Washington, D.C.
- Arnett RH Jr. 2000. American Insects: A Handbook of the Insects of America North of Mexico. CRC Press. 2nd Ed. Boca Raton, Florida. 1003 pp.
- Austin DF. 2004. Florida Ethnobotany. CRC Press. Boca Raton, Florida. 909 pp.
- Barbier R, Chauvin G. 1976. L'enveloppe vitelline chez les Lépidoptères: Transformation lors de l'activation de l'ovocyte et liaison avec la cuticule sérosale. Bulletin de la Société Zoologique de France 101: 1084-1085.
- Berenbaum MR, Moreno B, Green E. 1992. Soldier bug predation on swallowtail caterpillars (Lepidoptera: Papilionidae): Circumvention of defensive chemistry. Journal of Insect Behavior 5: 547-553.
- Blatchley WS. 1896. Miscellaneous notes. Canadian Entomologist 28: 265-266.
- Borror DJ. 1960. Dictionary of Word Roots and Combining Forms. Mayfield Publishing Company. Palo Alto, California. 134 pp.
- Brock JP. Kaufman K. 2003. Butterflies of North America. Houghton Mifflin. New York, New York. 383 pp.
- Brower JV. 1958. Experimental studies of mimicry in some North American butterflies. 2. Battus philenor and Papilio troilus, P. polyxenes and P. glaucus. Evolution 12: 123-136.
- Brower LP, Brower JVZ. 1962. The relative abundance of model and mimic butterflies in natural populations of the Battus philenor mimicry complex. Ecology 43: 154-158.
- Burgess KS, Singfield J, Melendez V, Kevan PG. 2004. Pollination biology of Aristolochia grandiflora (Aristolochiaceae) in Veracruz, Mexico. Annals of the Missouri Botanical Garden 91: 346-356.
- Cech R, Tudor G. 2005. Butterflies of the East Coast. Princeton University Press. Princeton, New Jersey. 345 pp.
- Chen Z, Zhu D. 1987. Aristolochia alkaloids. pp. 29-65. In: Brossi A. (ed.). The Alkaloids: Chemistry and Pharmacology. Vol. 31. Academic Press. New York.
- Codella SG Jr., Lederhouse RC. 1989. Intersexual comparison of mimetic protection in the black swallowtail butterfly, Papilio polyxenes: Experiments with captive blue jay predators. Evolution 43: 410-420.
- Comstock JA, Grimshawe FM. 1935. Early stages of Papilio polydamas lucayus R. & J. Bulletin of the Southern California Academy of Sciences 34: 76-80.
- Crosswhite FS, Crosswhite CD. 1985. The southwestern pipevine (Aristolochia watsonii) in relation to snakeroot oil, swallowtail butterflies, and ceratopogonid flies. Desert Plants 6: 203-207.
- Culley TM, Klooster MR. 2007. The cleistogamous breeding system: A review of its frequency, evolution, and ecology in angiosperms. The Botanical Review, 73:1-30.
- Dimarco RD, Fordyce JA. 2013. Larger clutches of chemically defended butterflies reduce egg mortality: Evidence from Battus philenor. Ecological Entomology 38: 535-538.
- Dimarco RD, Nice CC, Fordyce JA. 2012. Family matters: Effect of host plant variation in chemical and mechanical defenses on a sequestering specialist herbivore. Oecologia 170: 687-693.
- Doucet SM, Meadows MG. 2009. Iridescence: A functional perspective. Journal of the Royal Society Interface (Interface Focus Supplement) 6: S115-S132.
- Duke JA. 2001. CRC Handbook of Medicinal Herbs. CRC Press. Boca Raton, Florida. 677 pp.
- Eisner T. 2003. Chapter 2. Vinegaroons and other wizards. pp. 44-73. In: For Love of Insects. The Belknap Press of Harvard University Press. Cambridge, Massachusetts. 448 pp.
- Eisner T, Eisner M, Siegler M. 2005a. Chapter 9. Class Diplopoda, Order Polydesmida, Family Polydesmidae, Apheloria kleinpeteri, a polydesmid millipede. pp. 43-47. In: Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-legged Creatures. Harvard University Press. Cambridge, Massachusetts. 372 pp.
- Eisner T, Eisner M, Siegler M. 2005b. Chapter 64. Class Insecta, Order Lepidoptera, Family Papilionidae, Eurytides marcellus, the zebra swallowtail butterfly. pp. 297-303. In: Secret Weapons: Defenses of Insects, Spiders, Scorpions, and Other Many-legged Creatures. Harvard University Press. Cambridge, Massachusetts. 372 pp.
- Eisner T, Kluge AF, Ikeda MI, Meinwald YC, Meinwald J. 1971. Sesquiterpenes in the osmeterial secretion of a papilionid butterfly, Battus polydamas. Journal of Insect Physiology 17: 245-250.
- Eisner T, Meinwald YC. 1965. Defensive secretion of a caterpillar (Papilio). Science 150: 1733-1735.
- Feeny P. 1991. Chemical constraints on the evolution of swallowtail butterflies. pp. 315-340. In: Price PW, Lewinsohn TM, Fernandez GW, Benson WW. (eds.). Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. John Wiley & Sons. New York. 639 pp.
- Field WD. 1938. A manual of the butterflies and skippers of Kansas (Lepidoptera: Rhopalocera). Bulletin of the University of Kansas: Biological Series 39: 199.
- Flora of North America. Undated. Vol. 3. [http://www.efloras.org/volume_page.aspx?volume_id=1003]) (23 December 2016)
- Glassberg J, Minno MC, Calhoun JV. 2000. Butterflies Through Binoculars: A Field, Finding, and Gardening Guide to Butterflies in Florida. Oxford University Press. New York. 242 pp.
- Hall DW, Brown BV. 1993. Pollination of Aristolochia littoralis (Aristolochiales: Aristolochiaceae) by males of Megaselia spp. (Diptera: Phoridae). Annals of the Entomological Society of America 86: 609-613.
- Hazel WN. 1995. The causes and evolution of phenotypic plasticity in pupal color in swallowtail butterflies. pp. 205-210. In: Scriber JM, Tsubake Y, Lederhouse RC. (eds). Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Inc. Gainesville, Florida. 459 pp.
- Hazel WN, West DA. 1979. Environmental control of pupal color in swallowtail butterflies (Lepidoptera: Papilionidae) Battus philenor (L.) and Papilio polyxenes Fabr. Ecological Entomology 4: 393-400.
- Hazel WN, West DA. 1983. The effect of larval photoperiod on pupal color and diapause in swallowtail butterflies. Ecological Entomology 8: 37-42.
- Heinrich M, Chan J, Wanke S, Neinhuis C, Simmonds JSJ. 2009. Local uses of Aristolochia species and content of nephrotoxic aristolochic acid 1 and 2 - a global assessment based on bibliographic sources. Journal of Ethnopharmacology 125: 108-144.
- Heitzman JR, Heitzman JE. 1987. Butterflies and Moths of Missouri. Missouri Department of Conservation. Jefferson, Missouri. 385 pp.
- Howe WH. 1975. The Butterflies of North America. Doubleday. Garden City, NJ. 633 pp.
- Howe WH. 1988. Larval predation of the blue swallowtail, Battus philenor, in Kansas. News of the Lepidopterists’ Society No. 4, p.61.
- Iftner D, Shuey JA, Calhoun JV. 1992. Butterflies and Skippers of Ohio. College of Biological Sciences. The Ohio State University. Columbus, Ohio. 212 pp.
- Kandori I, Tsuchihara K, Suzuki TA, Yokoi T, Papaj DR. July 29, 2015. Long frontal projections help Battus philenor (Lepidoptera: Papilionidae) larvae find host plants. PLOS ONE 10: e0131596.
- Kendall RO. 1964. Larval foodplants for twenty-six species of Rhopalocera (Papilionoidea) from Texas. Journal of the Lepidopterists’ Society 18: 129-157.
- Layberry RA, Hall PW, Lafontaine JD. 1998. The Butterflies of Canada. University of Toronto Press. Toronto, Canada. 280 pp.
- Lederhouse RC. 1995. Comparative mating behavior and sexual selection in North American swallowtail butterflies. pp. 117-131. In: Scriber JM, Tsubaki Y, Lederhouse RC. eds. Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Inc. Gainesville, Florida. 459 pp.
- Linnaeus C. 1771. Regni animalis, Appendix. Insecta, pp. 529-543. In: Mantissa plantarum altera generum editionis VI & specierum editionis II. Laurentius Salvius. Stockholm, Sweden. pp. 143-588. [description of Papilio philenor, p. 535] (3 February 2017)
- Miller JS. 1987. Phylogenetic studies in the Papilioninae (Lepidoptera: Papilionidae). Bulletin of the American Museum of Natural History 186: 365-512.
- Minno MC, Butler JF, Hall DW. 2005. Florida Butterfly Caterpillars and their Host Plants. University Press of Florida. Gainesville, Florida. 341 pp.
- Minno MC, Minno M. 1999. Florida Butterfly Gardening. University Press of Florida. Gainesville, Florida. 210 pp.
- Moerman DE. 1998. Native American Ethnobotany. Timber Press, Portland, Oregon. 927 pp.
- Mondal, P. 2016. Pollination in plants: Types, advantages and disadvantages. (23 March 2020)
- Nechaev VA, Nakonechnaya OV. 2009. Structure of fruits and seeds and ways of dissemination of two species of the genus Aristolochia L. in Primorsky Krai. Biology Bulletin 36: 393-396.
- Nice RW, Fordyce JA. 2006. How caterpillars avoid overheating: Behavioral and phenotypic plasticity of pipevine swallowtail larvae. Oecologia 146: 541-548.
- Nielsen ME, Papaj DR. 2015. Effects of developmental change in body size on ectotherm body temperature and behavioral thermoregulation: Caterpillars in a heat-stressed environment. Oecologia 177: 171-179.
- Odendaal FJ, Rausher MD, Benrey B, Nunez-Farfan J. 1987. Predation by Anolis lizards on Battus philenor raises questions about butterfly mimicry systems. Journal of the Lepidopterists’ Society 41: 141-143.
- Ômura H, Honda K, Feeny P. 2006. From terpenoids to aliphatic acids: Further evidence for late-instar switch in osmeterial defense as a characteristic trait of swallowtail butterflies in the tribe Papilonini. Journal of Chemical Ecology 32: 1999-2012.
- Opler PA, Krizek GO. 1984. Butterflies East of the Great Plains. The Johns Hopkins University Press. Baltimore, MD. 294 pp.
- Opler PA, Malikul V. 1998. A Field Guide to Eastern Butterflies. Peterson Field Guides. Houghton Mifflin Company. New York. 486 pp.
- Osorio R. 2010. Ant dispersal in Aristolochia serpentaria (Virginia snakeroot). (23 March 2020)
- Otis GW, Locke B, McKenzie NG, Cheung D, MacLeod E, Careless P, Kwoon A. 2006. Local enhancement in mud-puddling swallowtail butterflies (Battus philenor and Papilio glaucus). Journal of Insect Behavior 19: 685-696.
- Papaj DR. 1986a. An oviposition mistake by Battus philenor L. (Papilionidae). Journal of the Lepidopterists’ Society 40: 348-349.
- Papaj DR. 1986b. Leaf buds, a factor in host selection by Battus philenor butterflies. Ecological Entomology 11: 301-307.
- Papaj DR, Newsom GM. 2005. A within-species warning function for an aposematic signal. Proceedings of the Royal Society B – Biological Sciences 272: 2519-2523.
- Pegram KV, Han HA, Rutowski RL. 2015. Warning signal efficacy: Assessing the effects of color, iridescence, and time of day in the field. Ethology 121: 861-873.
- Pegram KV, Lillo MJ, Rutowski RL. 2013. Iridescent blue and orange components contribute to the recognition of a multicomponent warning signal. Behaviour 150: 321-336.
- Pegram KV, Rutowski RL. 2014. Relative effectiveness of blue and orange warning colours in the contexts of innate avoidance, learning and generalization. Animal Behaviour 92: 1-8.
- Pegram KV, Rutowski RL. 2016. Effects of directionality, signal intensity, and short-wavelength components on iridescent warning signal efficacy. Behavioral Ecology and Sociobiology 70: 1331-1343.
- Pelham JP. 2008. A catalogue of the butterflies of the United States and Canada. Lepidoptera Research Foundation. Beverly Hills, California. 658 pp.
- Pfeifer HW. 1966. Revision of the North and Central American hexandrous species of Aristolochia (Aristolochiaceae). Annals of the Missouri Botanical Garden 53: 115-196.
- Pinto CF, Troncoso AJ, Urzúa A, Niemeyer M. 2009b. Use of volatiles of Aristolochia chilensis (Aristolochiaceae) in host searching by fourth-instar larvae and adults of Battus polydamas archidamas (Lepidoptera: Papilionidae: Troidini). European Journal of Entomology 106: 63-68.
- Poulton EB. 1909. Mimicry in butterflies of North America. Annals of the Entomological Society of America 11: 203-242.
- Proctor M, Yeo P, Lack A. 1996. The Natural History of Pollination. Timber Press. Portland, Oregon. 479 pp.
- Racheli T, Oliverio M. 1993. Biogeographical patterns of the neotropical genus Battus Scopoli 1777 (Lepidoptera: Papilionidae). Tropical Zoology 6: 55-65.
- Rausher MD. 1978. Search image for leaf shape in a butterfly. Science 200: 1071-1073.
- Rausher MD. 1979. Egg recognition: Its advantage to a butterfly. Animal Behaviour 27: 1034-1040.
- Rausher MD, Feeny P. 1980. Herbivory, plant density, and plant reproductive success: The effect of Battus philenor on Aristolochia reticulata. Ecology 61: 905-917.
- Richards OW, Davies RG. 1977. Chap. 18 Embryology. In: Vol. 1. Imms’ Textbook of Entomology. 10th edition. John Wiley & Sons. New York. 418 pp.
- Robinson GS, Ackery PR, Kitching IJ, Beccaloni GW, Hernández LM. Undated. HOSTS - a Database of the World's Lepidopteran Hostplants. (23 March 2020)
- Rutowski RL, Rajyaguru PK. 2013. Male-specific iridescent coloration in the pipevine swallowtail butterfly (Battus philenor) is used in mate choice by females but not sexual discrimination by males. Journal of Insect Behavior 26: 200-211.
- Rutowski RL, Nahm AC, Macedonia JM. 2010. Iridescent hindwing patches in the pipevine swallowtail: Differences in dorsal and ventral surfaces relate to signal function and context. Functional Ecology 24: 767-775.
- Sachdev-Gupta K, Feeny PP, Carter M. 1993. Oviposition stimulants for the pipevine swallowtail butterfly, Battus philenor (Papilionidae), from an Aristolochia host plant: synergism between inositols, aristolochic acids and a monogalactosyl diglyceride. Chemoecology 4: 19-28.
- Schaneberg, BT, Applequist WL, Khan IA. 2002. Determination of aristolochic acid I and II in North American species of Asarum and Aristolochia. Pharmazie 57: 686-689.
- Schull EM. 1987. The Butterflies of Indiana. Indiana Academy of Science (Distributed by Indiana University Press). Bloomington, Indiana. 262 pp.
- Scopoli, G.A. 1777. Introductio ad historiam naturalem sistens genera lapidum, plantarum, et animalium: hactenus detecta, caracteribus essentialibus donata, in tribus divisa, subinde ad leges naturae. Apud Wolfgangum Gerle. Prague. p. 433. (23 March 2020)
- Scott JA. 1986. The Butterflies of North America. Stanford University Press. Stanford, California. 583 pp.
- Sime K. 2000. Two new records of pimpline ichneumonids attacking Battus philenor (Linnaeus) (Lepidoptera: Papilionidae). Journal of Hymenoptera Research 9: 210-212.
- Sime K. 2002. Chemical defense of Battus philenor larvae against attack by the parasitoid Trogus pennator. Ecological Entomology 27: 337-345.
- Sime K, Feeny PP, Haribal MM. 2000. Sequestration of aristolochic acids by the pipevine swallowtail, Battus philenor (L.): Evidence and ecological implications. Chemoecology 10: 169-178.
- Sims SR, Shapiro AM. 1983a. Pupal color dimorphism in California Battus philenor (L.) (Papilionidae): Mortality factors and selective advantage. Journal of the Lepidopterists’ Society 37: 236-243.
- Sims SR, Shapiro AM. 1983b. Pupal color dimorphism in California Battus philenor: Pupation sites environmental control, and diapause linkage. Ecological Entomology 8: 95-104.
- Smith JE. 1797. Natural History of the Rarer Lepidopterous Insects of Georgia. Vol. 1. Bensley, London. 100 pp. (From the observations of John Abbot)
- Sourakov A, Daniels JC. 2002. Is Battus philenor hirsuta a subspecies? News of the Lepidopterists’ Society 44: 64-65.
- Stamp NE. 1986. Physical constraints of defense and response to invertebrate predators by pipevine caterpillars (Battus philenor: Papilionidae). Journal of the Lepidopterists’ Society 40: 191-205.
- Stehr FW. 1987. Immature Insects. Kendall/Hunt. Dubuque, Iowa. 754 pp.
- Tyler HA. 1975. The Swallowtail Butterflies of North America. Naturegraph Publishers. Healdsburg, CA. 192 pp.
- Tyler HA, Brown KS Jr., Wilson KH. 1994. Swallowtail Butterflies of the Americas. Scientific Publishers. Gainesville, Florida. 376 pp.
- Underwood DLA. 1994. Methods for sexing Lepidoptera larvae using external morphology. Journal of the Lepidopterists’ Society 48: 258-263. (includes Battus philenor)
- Wagner DL. 2005. Caterpillars of Eastern North America. Princeton University Press. Princeton, New Jersey. 512 pp.
- West DA. 1983. Hand pairing of Battus philenor (Papilionidae). Journal of the Lepidopterists’ Society 37: 90.
- West DA, Hazel WN. 1979. Natural pupation sites of swallowtail butterflies (Lepidoptera: Papilioninae): Papilio polyxenes Fabr., P. glaucus L. and Battus philenor (L.). Ecological Entomology 4: 387-392.
- Weintraub JD. 1995. Host plant association patterns and phylogeny in the tribe Troidini (Lepidoptera: Papilionidae). pp. 307-316. In: Scriber JM, Tsubake Y, Lederhouse RC. eds. Swallowtail Butterflies: Their Ecology and Evolutionary Biology. Scientific Publishers, Inc. Gainesville, Florida. 459 pp.
- Zomlefer WB. 1994. Plant Families. The University of North Carolina Press. Chapel Hill, North Carolina. 430 pp.















![Elegant Dutchman's pipe or calico flower, Aristolochia elegans M.T. Mast [synonym: Aristolochia littoralis Parodi]), a cultured exotic which is a death trap for the pipevine swallowtail caterpillar, Battus philenor (L.)](../BFLY/Battus_philenor19.jpg)




![Early instar long-tailed skipper (Urbanus proteus [L.]) leaf shelter on woolly pipevine, Aristolochia tomentosa Sims. Inset: leaf shelter opened to show larva](../BFLY/Battus_philenor26.jpg)