common name: Asian chestnut gall wasp
scientific name: Dryocosmus kuriphilus Yasumatsu (Insecta: Hymenoptera: Cynipoidea: Cynipidae)
Introduction - Distribution - Description - Life Cycle - Hosts - Damage - Management - Selected References
Introduction (Back to Top)
Gall wasps (Hymenoptera: Cynipidae) are insects that interact with plants and induce specialized plant tissue structures called galls. Galls house the immature stages and provide them protection from natural enemies. Most cynipids are not considered economic pests (Stone et al. 2002). A notable exception is Dryocosmus kuriphilus Yasumatsu, also called the Asian chestnut gall wasp, which induces galls mostly on leaves and buds of all chestnut species (Castanea spp., Fagales: Fagaceae). This wasp is considered the most important pest of chestnut trees throughout the world (Moriya et al. 1989, Brussino et al. 2002, Aebi et al. 2006). Originally from China, Dryocosmus kuriphilus was accidentally introduced in the USA in 1974 (Bernardo et al. 2013). Since its first recorded sighting in Fort Valley (Peach County), Georgia, the wasp spread northward, and now covers nearly 1.5 million square kilometers. It has not yet been recorded in Florida, but movement of infested plant material for nut production or ornamental purposes and natural dispersal by flight represent a risk of potential invasion in the state.
Figure 1. Galls induced by Dryocosmus kuriphilus. Photograph by Emilie P. Demard, University of Florida.
Distribution (Back to Top)
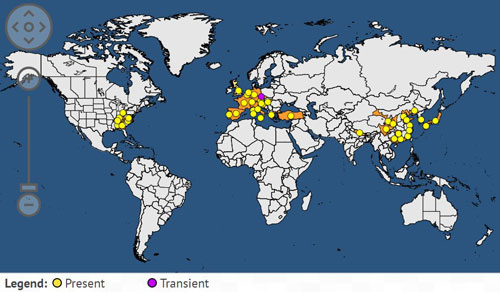
Originally from China, Dryocosmus kuriphilus was first reported in Japan in 1941 and then observed in Korea in 1958. It was accidentally introduced into the USA in 1974, where it was detected in Georgia on Chinese chestnut (Castanaea mollissima) after the importation of infested plant material by a private grower (Rieske 2007). Within ten years, it damaged local chestnut orchards with Chinese and Japanese chestnut varieties (Aebi et al. 2006). It was more recently detected in northern Nepal in 1999. In Europe, it was first recorded in the Piedmont region of Italy in 2002 (Aebi et al. 2007). Since 2002, Dryocosmus kuriphilus has spread throughout Italy to France and Slovenia in 2005 (Aebi et al. 2006), Hungary and Switzerland in 2009, Croatia and Netherlands in 2010, Slovakia, the Czech Republic, and Spain in 2012, then Turkey and Portugal in 2014 (European Food Safety Authority 2010, Gibbs et al. 2011, Borowiec et al. 2014).
Figure 2. Worldwide distribution of Dryocosmus kuriphilus. Map from the European and Mediterranean Plant Protection Organization Global database, 2017. (Available online at: https://gd.eppo.int/taxon/DRYCKU/distribution).
In the USA, the gall wasp is known to occur in Georgia, Alabama, North Carolina, Tennessee, Virginia, Kentucky, Ohio, Maryland and Pennsylvania (Rieske 2007). In Florida, Dryocosmus kuriphilus has never been reported. However, chestnuts can be grown in north Florida, and it could represent an alternative crop for growers who are looking to diversify their production. Climate change and global warming may also create favorable conditions for the growth of this crop in Florida. Thus, the Asian chestnut gall wasp may become a future threat for the state.
Description (Back to Top)
Eggs: The eggs of Dryocosmus kuriphilus are oval, milky white, and 0.1-0.2 mm long with a long stalk.
Larvae: The larvae are eyeless and legless. They measure about 2.5 mm long when fully grown. Their color is generally milky white.
Figure 3. Larvae of Dryocosmus kuriphilus. Photograph by Hélina Deplaude, Chambre d’Agriculture d’Ardèche.
Pupae: The pupae are 2.5 mm long, black or dark brown.
Figure 4. Pupa of Dryocosmus kuriphilus. Photograph by Hélina Deplaude, Chambre d’Agriculture d’Ardèche.
Adult: The adult female is 2.5 to 3.0 mm long on average and has a black body. The wings are membranous and translucent, and the legs are orange to brown (European and Mediterranean Plant Protection Organization 2005). Males are unknown.
Figure 5. Adult female of Dryocosmus kuriphilus. Photograph by Hélina Deplaude, Chambre d’Agriculture d’Ardèche.
Life Cycle (Back to Top)
The Asian chestnut gall wasp is a univoltine species, that is, it only produces one generation per year. It reproduces by thelytokous parthenogenesis (reproduction is achieved without mating and the unfertilized egg develops into a female). Sexual reproduction has never been observed. This results in a population entirely composed of female clones (Bernardo et al. 2013).
Figure 6. Cross section of a gall on Castanea sp. containing several larval chambers of Dryocosmus kuriphilus, (the coin shown is similar in size to a US nickel). Photograph by Emilie P. Demard, University of Florida.
Adults emerge in early summer, and females lay eggs immediately inside chestnut twig buds. The eggs hatch within 30 to 40 days. First instars overwinter and remain in this stage until spring. At bud burst in spring, larval activity resumes, causing the formation of green or rose-colored galls (Borowiec et al. 2014). Each gall can potentially contain more than 16 larval chambers. An average of 3.5 larvae per gall is observed, but this number varies greatly depending on gall wasp pressure, bud size, and chestnut variety (Kato and Hijii 1997, Cooper and Rieske 2011, Panzavolta et al. 2013). After activity resumes, the larva lives in the gall for 30 to 70 days (50 days on average) (Ding et al. 2004).
The fully developed adult females remain in the gall for 10 to 15 days before emerging (Gibbs et al. 2011). When adults emerge during June-July, they leave an exit hole in the gall. An adult can live up to ten days and lay more than 100 eggs during this time (European and Mediterranean Plant Protection Organization 2005). After adult emergence, galls usually become dry, brown, and woody over time. Dried galls can remain on the tree for several years.
Figure 7. Dried galls of Dryocosmus kuriphilus during fall. Photograph by Emilie P. Demard, University of Florida.
Hosts (Back to Top)
Dryocosumus kuriphilus is a global pest of chestnuts, Castanea spp. (Fagales: Fagaceae). More particularly, it is reported to attack the Japanese chestnut, Castanea crenata, the American chestnut, Castanea dentata, the Chinese chestnut Castanea mollissima, the European chestnut Castanea sativa, and their hybrids. It also infests the Seguin chestnut, Castanea seguinii, in China (European and Mediterranean Plant Protection Organization 2005).
Damage (Back to Top)
Dryocosmus kuriphilus is the most severe insect pest of chestnut (European and Mediterranean Plant Protection Organization 2005). It can reduce wood production and fruit yield by 50 to 75% (Aebi et al. 2007). However, these numbers can vary depending on the susceptibility of the cultivars to the pest.
The galls induced by Dryocosmus kuriphilus can be localized on the growing twigs, the leaf petiole or midrib, the stipules, or the buds of chestnut trees. The galls start at approximately 5 to 20 mm in diameter and can grow up to 4 cm in diameter as the leaf tries to form (Ding et al. 2004).
Figure 8. Different types of galls induced by Dryocosumus kuriphilus: (a) on the midrib of the leaf, (b) on the bud, (c) on the stipule, (d) on the growing twig. Photographs by Emilie P. Demard, University of Florida.
Dryocosmus kuriphilus may reduce yield of chestnuts through both direct and indirect mechanisms. Galls can directly prevent the formation of the female flower when they form on the apical buds of the shoots, which stops shoot growth and causes flower abortion. Also, yield can be indirectly affected as a result of reduced leaf area, photosynthesis, and tree biomass within the years following the infestation (Kato and Hijii 1997). Galls can cause a progressive decrease in tree vigor; dieback of twigs is often noticed. Severe infestations may result in death of chestnut trees. A study highlighted the fact that the wasp increases tree susceptibility to chestnut blight damage, Cryphonectria parasitica Murr (Diaporthales: Valsaceae), by by weakening plant vigor (Turchetti et al. 2010).
Management (Back to Top)
Visual inspection for detection of the presence of the wasp is only effective after May or June when visible galls appear as a result of larval feeding within the buds. Surveillance for galling symptoms in April, May, or June allows for early detection and destruction of affected plants before the emergence of the adult stage (European Food Safety Authority 2010).
Phytosanitary measures: The Asian chestnut gall wasp was added to the European and Mediterranean Plant Protection Organization (EPPO) A2 action list in 2003, and EPPO member countries are recommended to regulate it as a quarantined pest. Introduction of the pest from Asia and America is effectively prevented since the import of all plants of Castanea (except fruits and seeds) from non-European countries is prohibited by most EPPO countries on account of other pests. Within the EPPO region, young plants or shoots of Castanea used for grafting and coming from infested areas should be produced in a place kept free from Dryocosmus kuriphilus (European and Mediterranean Plant Protection Organization 2005).
Chemical control: The use of chemicals against the immature stages is inefficient because of the cryptic nature of the insect living in dormant buds for the majority of its life; the larva is protected within the plant tissues (Moriya et al. 1989, Cooper and Rieske 2007). The effectiveness of chemical control of adults relies on the detection of adult emergence for an appropriately timed treatment. Also, the efficiency of this method is reduced by the difficulty of covering the entire canopy of the tree when using ground-based applicators (Bernardo et al. 2013). In addition, chemical treatments are often impractical due to the natural distribution of chestnut trees on steep terrain (Germinara et al. 2011).
Host plant resistance: The breeding of resistant chestnut varieties of Castanea crenata was successful for approximately 20 years in Japan, but plant resistance was overcome by a novel virulent biotype of Dryocosmus kuriphilus in the early 1970s (Murakami 1981). In addition, the mode of inheritance of resistance was not established, which limited the application of more rapid contemporary methods for selection. In the USA, Payne (1978) observed chinquapins (Castanea pumila and Castanea alnifolia) seemingly resistant or immune to the wasp, but the source of resistance remains unclear. Moreover, an Euro-Japanese hybrid (Castanea sativa x Castanea crenata) called Bouche de Bétizac has been identified as completely resistant to Dryocosmus kuriphilus. Dini et al. (2012) suggest the hybrid avoids infestation through a hypersensitive reaction that kills the first instar.
Developing resistant varieties is a potential management option, but several limits are noted. Indeed, it is only beneficial for new plantings and will not help existing chestnut plantations. There is also the risk of selection of Dryocosmus kuriphilus biotypes that may overcome plant resistance, and the level of resistance of the hybrid varieties is still poorly known. Finally, the selection for resistance is a slow process, which prevents its development in the short term (European Food Safety Authority 2010).
Classical biological control: To date, the most effective method to control the Asian chestnut gall wasp is the introduction of the parasitoid Torymus sinensis Kamijo (Hymenoptera: Torymidae). This chalcid wasp, native to China, shows a high host specificity, and its life cycle matches that of its host. These two traits are essential for a successful biological control agent (Aebi et al. 2006). It was first released in Japan in 1975 where it reduced the infestation level from 43% to 3% within five years, far below the tolerable threshold of 30% of infested shoots (Moriya et al. 1989). Infestation has stayed low since 1982 in Japan. Similarly, the parasitoid was released in Georgia, USA in 1977. Its introduction in Europe was initiated in Italy in 2005 and in France in 2011 (Cooper and Rieske 2007, Quacchia et al. 2007, Moriya 2008, Borowiec et al. 2014). The parasitoid was also introduced to Hungary and Croatia in 2014 and Slovenia in 2015 (Radocz et al. 2015).
Figure 9. The parasitoid Torymus sinensis on a gall of Dryocosmus kuriphilus. Photograph by Hélina Deplaude, Chambre d’Agriculture d’Ardèche.
Torymus sinensis is univoltine like its host. It essentially reproduces amphigonically (sexual reproduction), but it may reproduce by arrhenotokous parthenogenesis (unfertilized eggs develop into males and fertilized eggs develop into females). According to Cooper and Rieske (2011), this wasp is a generalist parasitoid of cynipid gall wasps in China but acts as a specialist when Dryocosmus kuriphilus galls are found in abundance such as in Japan and possibly North America. While Zhang (2009) claims that Torymus sinensis is monophagous (eats only one type of food), the European and Mediterranean Plant Protection Organization (2010) concluded that the parasitoid seems to be monophagous or highly oligophagous (i.e., it has a very limited host range).
Females of Torymus sinensis lay eggs into newly formed galls either on the body of the host larva or on the wall of the larval chamber (Quacchia et al. 2007). Usually, one egg per host is laid, but under natural conditions, multiple eggs per larva have been observed in a single chamber. However, only one parasitoid larva completes its development because of cannibalism among young Torymus sinensis larvae. After hatching, the larva feeds ectoparasitically on the host’s mature larva until pupation and stays in the gall during winter. Adult wasps emerge in spring (early April) from the withered galls, synchronously with sprouting of chestnut trees and gall initiation. Female Torymus sinensis are able to parasitize newly developing galls for a period of several weeks (Quacchia et al. 2007, European Food Safety Authority 2010, Cooper and Rieske 2011, Ferracini et al. 2015).
Figure 10. Laboratory rearing of Torymus sinensis for classical biological control of Dryocosmus kuriphilus. Photograph by Emilie P. Demard, University of Florida.
The adult of Torymus sinensis has several morphological criteria that help to distinguish it from other parasitoids. The tarsi are divided into five tarsomeres, the long ovipositor projects over the female abdomen, the hind coxae are large, there is a carina on the scutellum, and the body color is shiny metallic green (Institut National de la Recherche Agronomique 2012).
Figure 11. Morphological criteria for the identification of Torymus sinensis. Photograph by Hélina Deplaude, Chambre d’Agriculture d’Ardèche.
Although classical biological control is the most effective method to control Dryocosmus kuriphilus, ongoing studies attempt to assess the environmental risks of using Torymus sinensis. The conditions under which Torymus sinensis could target alternate hosts and the likelihood of its hybridization with native Torymus species are central issues for a successful biological control program (Aebi et al. 2007, Gibbs et al. 2011). For instance, hybridization between the native parasitoid Torymus beneficus and Torymus sinensis has been clearly shown in Japan, but the biological impact is still hard to predict (Aebi et al. 2007, Yara 2014). Thus, molecular studies are ongoing in Japan, Italy, and France to identify cryptic lineages and characterize the native parasitoid community (Aebi et al. 2007, Borowiec et al. 2014).
Conservation or augmentation biological control: The rapid recruitment of native oak and rose cynipid parasitoids to Dryocosmus kuriphilus suggests a potential value of native parasitoids to be used as biological control agents in augmentative biological control programs. Eighteen parasitoid species associated with Japanese oak gall wasps also attack Dryocosmus kuriphilus, representing host shifts from native hosts to the invader. In Korea, Dryocosmus kuriphilus recruited a parasitoid assemblage of 15 chalcid species (Aebi et al. 2006). In the USA, two torymid species, Torymus advenus and Torymus tubicola, were recorded by Payne (1978). Cooper and Riesk (2011) identified Ormyrus labotus as a parasitoid of Dryocosmus kuriphilus. In Italy, 16 native parasitoid species belonging to six families (Eurytomidae, Pteromalidae, Torymidae, Eupelmidae, Eulophidae, and Ormyridae) were recorded (Aebi et al. 2006).
Figure 12. Oak cynipid parasitoids: (a) Mesopolopus sp., (b) Megastigmus sp., (c) Eupelmus sp. Photographs by Emilie P. Demard, University of Florida.
Although oak cynipid parasitoids provide potential for biological control, the mismatch between the emergence of native parasitoids and Dryocosmus kuriphilus results in low attack rates (between 0.5 and 1.6%) compared to the well-synchronized parasitoid Torymus sinensis (Aebi et al. 2007). Also, further studies on the taxonomy and biology of these parasitoids is needed to improve our understanding of interactions between the invasive gall wasp, the introduced parasitoid, and the native parasitoids. Indeed, several species were identified as competitors or facultative hyperparasitoids (parasitoid acting either as a primary parasitoid or a secondary parasitoid). For example, Eurytoma brunniventris is known to be polyphagous and to feed on gall tissues, and Eupelmus urozonus may be a facultative hyperparasitoid (Quacchia et al. 2013, Francati et al. 2015). Both were observed to have an important role in the delay in the population increase of the introduced Torymus sinensis in some areas of Japan (Murakami et al. 1995). Cooper and Riesk (2011) also recorded Ormyrus labotus, which is a native parasitoid of oak galling cynipids in the USA and a hyperparasitoid of Torymus sinensis.
Selected References (Back to Top)
- Aebi A, Schönrogge K, Melika G, Alma A, Bosio G, Quacchia A, Picciau L, Abe Y, Moriya S, Yara K, Seljak G, Stone GN. 2006. Parasitoid recruitment to the globally invasive chestnut gall wasp Dryocosmus kuriphilus. p. 103-121. In: Ozaki K, Yukawa J, Ohgushi T, Price PW (Editor). Ecology and Evolution of Galling Arthropods and Their Associates. Springer-Verlag, Tokyo, Japan.
- Aebi A, Schönrogge K, Melika G, Quacchia A, Alma A, Stone GN. 2007. Native and introduced parasitoids attacking the invasive chestnut gall wasp Dryocosmus kuriphilus. EPPO Bulletin 37: 166-171.
- Bernardo U, Iodice L, Sasso R, Tutore VA, Cascone P, Guerrieri E. 2013. Biology and monitoring of Dryocosmus kuriphilus on Castanea sativa in southern Italy. Agriculture and Forest Entomology 15: 65-76.
- Borowiec N, Thaon M, Brancaccio L, Warot S, Vercken E, Fauvergue X, Ris N, Malausa J-C. 2014. Classical biological control against the chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera, Cynipidae) in France. Plant Protection Quarterly 29: 7-10.
- Brussino G, Bosio M, Giordano R, Ramello F, Melika G. 2002. A dangerous exotic insect threating European chestnut. Information Agrario 58: 59-61.
- Cooper WR, Rieske LK. 2007. Community associates of an exotic gallmaker, Dryocosmus kuriphilus (Hymenoptera: Cynipidae), in eastern North America. Annals of the Entomological Society of America 100: 236-244.
- Cooper WR, Rieske LK. 2011. A native and an introduced parasitoid utilize an exotic gallmaker host. BioControl 56: 725-734.
- Ding Y, Bi S, Fang G, He L. 2004. Relationship between occurrence of Dryocosmus kuriphilus and development of cecidum. Journal of Applied Ecology 15: 108-110.
- Dini F, Sartor C, Botta R. 2012. Detection of a hypersensitive reaction in the chestnut hybrid ‘Bouche de Bétizac’ infested by Dryocosmus kuriphilus Yasumatsu. Plant Physiology and Biochemistry 60: 67-73.
European and Mediterranean Plant Protection Organization. 2005. Dryocosmus kuriphilus. EPPO Bulletin 35: 422-424.
- European Food Safety Authority. 2010. Risk assessment of the oriental chestnut gall wasp, Dryocosmus kuriphilus for the EU territory and identification and evaluation of risk management options: Risk assessment of Dryocosmus kuriphilus. EFSA Journal 8: 1619.
- Ferracini C, Gonella E, Ferrari E, Saladini MA, Picciau L, Tota F, Pontini M, Alma A. 2015. Novel insight in the life cycle of Torymus sinensis, biocontrol agent of the chestnut gall wasp. BioControl 60: 169-177.
- Francati S, Alma A, Ferracini C, Pollini A, Dindo ML. 2015. Indigenous parasitoids associated with Dryocosmus kuriphilus in a chestnut production area of Emilia Romagna (Italy). Bulletinl of Insectology 68: 127-134.
- Germinara GS, Cristofaro AD, Rotundo G. 2011. Chemical cues for host location by the chestnut gall wasp, Dryocosmus kuriphilus. Journal of Chemical Ecology 37: 49-56.
- Gibbs M, Schönrogge K, Alma A, Melika G, Quacchia A, Stone GN, Aebi A. 2011. Torymus sinensis: a viable management option for the biological control of Dryocosmus kuriphilus in Europe? BioControl 56: 527-538.
- Institut National de la Recherche Agronomique. 2012. Critères morphologiques différenciant Torymus sinensis. In: Formation lutte biologique contre le cynips du châtaignier. Sophia Antipolis, France, 8.
- Kato K, Hijii N. 1997. Effects of gall formation by Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) on the growth of chestnut trees. Journal of Applied Entomology 121: 9-15.
- Moriya S. 2008. Chestnut gall wasp classical biological control in Japan. p. 835-837. In: Encyclopedia of Entomology. Springer, Dordrecht.
- Moriya S, Inoue K, Otake A, Shiga M, Mabuchi M. 1989. Decline of the chestnut gall wasp population, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) after the establishment of Torymus sinensis Kamijo (Hymenoptera: Torymidae). Applied Entomology and Zoology 24: 231-233.
- Murakami Y. 1981. The parasitoids of Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) in Japan and the introduction of a promising natural enemy from China (Hymenoptera: Chalcidoidea). Journal of the Faculty of Agriculture, Kyushu University 25: 167-174.
- Murakami Y, Ohkubo N, Moriya S, Gyoutoku Y, Kim HC, Kim KJ. 1995. Parasitoids of Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in South Korea with particular reference to ecologically different types of Torymus (Syntomaspis) sinensis (Hymenoptera: Torymidae). Applied Entomology and Zoology 30: 277-284.
- Panzavolta T, Bernardo U, Bracalini M, Cascone P, Croci F, Gebiola M, Iodice L, Tiberi R, Guerrieri E. 2013. Native parasitoids associated with Dryocosmus kuriphilus in Tuscany, Italy. ResearchGate 66: 195-201.
- Payne JA. 1978. Oriental chestnut gall wasp: New nut pest in North America. p. 86-88. In: Macdonald WL, Cech FC, Luchok J, Smith C (editor). Proceedings of the American Chestnut Symposium. West Virginia University Press, Morgantown.
- Quacchia A, Ferracini C, Nicholls JA, Piazza E, Saladini MA, Tota F, Melika G, Alma A. 2013. Chalcid parasitoid community associated with the invading pest Dryocosmus kuriphilus in north-western Italy. Insect Conservation and Diversity 6: 114-123.
- Quacchia A, Moriya S, Bosio G, Scapin I, Alma A. 2007. Rearing, release and settlement prospect in Italy of Torymus sinensis, the biological control agent of the chestnut gall wasp Dryocosmus kuriphilus. BioControl 53: 829-839.
- Radocz L, Melika G, Kovács G, Szilágyi A, Nagy M. 2015. Asian sweet chestnut gallwasp, Dryocosmus kuriphilus (Hymenoptera, Cypnidae): first record for Romania. North-Western Journal of Zoology (online first): art.157201.
- Rieske LK. 2007. Success of an exotic gallmaker, Dryocosmus kuriphilus, on chestnut in the USA: A historical account. EPPO Bulletin 37: 172-174.
- Stone GN, Schonrogge K, Atkinson RJ, Bellido D, Pujade-Villar J. 2002. The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annual Review of Entomology 47: 633-668.
- Turchetti T, Addario E, Maresi G. 2010. Interazioni tra cinipide galligeno e cancro della corteccia: Una nuova criticita per il castagno. Forest 7: 252-258.
- Yara K. 2014. Interaction between Torymus sinensis (Hymenoptera: Torymidae) and T. beneficus, introduced and indigenous parasitoids of the chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Japan Agricultural Research Quarterly 48: 35-40.
- Zhang Z. 2009. Study approaches on the chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu in China. Proceedings of the 4th International Chestnut Symposium. Acta Horticulturae 844: 425-432.